Heisenberg uncertainty principle

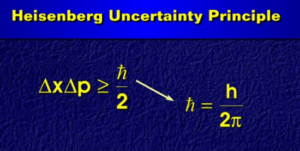
Heisenberg uncertainty principle Equation is provided here. If you want to get benefits from this post, you’ll love this post.
This post includes:
Heisenberg uncertainty principle:
- Definition
- Formula
- Equation
- Significance
- lot’s more
Keep reading
Heisenberg uncertainty principle Statement
The uncertainty principle is defined as: “It is impossible to measure simultaneously both the position and momentum of a microscopic particle with accuracy or certainty.”
When we are studying a large moving object say a planet, then we can follow its definition path on which it travels. If we know its initial position and momentum, then we can predict its position and momentum at any other time. But this is not possible for electrons, protons, and neutrons which are microscopic particles. Heisenberg has given a principle in this connection.
Uncertainty principle proof
In order to know the position of an object, we throw the photons of light upon them. If we want to have the idea for the position of the electron, then the photons of X -rays region have to be used because their wavelengths are very small and the possibility for the hitting of the electron is there. During this hitting, the photon transfers some of its energy to the electron. Therefore, the velocity and hence the momentum of the electron changes.
If we use the photons of longer wavelengths say of visible region, the velocity and the momentum will not change appreciably because longer wavelengths rarely find the chance to hit the electron. However, its position cannot be determined because the object will not be visible.
Keep in mind that, the uncertainty is not due to a lack of better techniques for the measurement of position and momentum. This is due to the reason that we cannot observe the microscopic objects without disturbing them. The uncertainty principle is not applicable to stationary electrons because, in the stationary state, the velocity of an electron is zero. As a result, the position of the electron can be accurately determined. However, both positions and velocities of an electron cannot be determined accurately.
Heisenberg uncertainty principle Equation
Let the motion of the particle is along the x-axis, then according to the de Broglie Hypothesis:
If the motion of the particle depends upon the three coordinates x,y,z the generalizing above relation, we have:
These are known as Heisenberg’s uncertainty relationships. According to these relationships: It is impossible to determine both the position and the momentum of a particle with ultimate precision.
The width Δx of the wave packet indicates the problem location of the particle, and Δpx is the range in momentum. So, the uncertainty principle may also be stated as:
“A particle cannot be described by a wave packet in which the position and momentum have arbitrary small ranges.”
It means that due to the wave nature, the exact position x of a particle cannot be determined, but it will be in the range Δx. Similarly, the true or exact momentum of the particle px cannot be determined, but it will be in the range of Δpx.
Watch also:
Related Topics:
- Schrodinger wave equation
- Laser
- What is a laser? How does it work?
- What are the different types of lasers?
- Uses of laser in daily life
- Helium (he) Neon (ne) laser working animation
- Properties and applications of laser light
Related Post
Recent Posts
Difference between Gastritis and ulcer
Gastritis and ulcers are irritations, which must be treated urgently in order not to develop…
Saudi Arabia launches new residency plans to draw foreign talent
On Thursday, Saudi Arabia unveiled fresh residency programs geared towards drawing in skilled professionals and…
Study in UAE: Your Complete Guide to Enrolling Abroad
Studying abroad can be a life-changing experience, opening doors to new cultures, perspectives, and opportunities.…
Difference between Osmosis and dialysis
The difference between osmosis and dialysis is that Osmosis is a physical phenomenon by…
Difference between Vitamins and proteins
The Difference between Vitamins and Proteins is given here. Vitamins and proteins are essential…
Difference between Windows and Linux
The Difference between Windows and Linux is given here. Windows and Linux are operating systems…
View Comments