First law of thermodynamics example

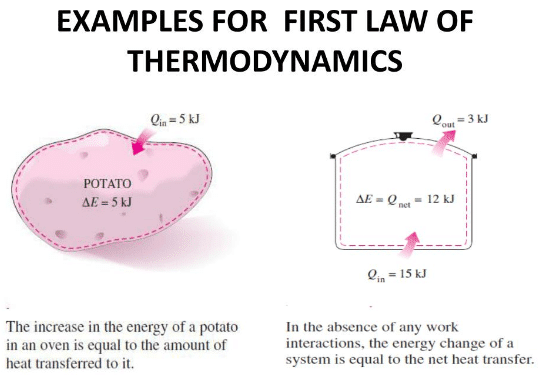


When heat is added to a system there is an increase in the internal energy due to the rise in temperature, an increase in pressure or change in the state. If at the same time, a substance is allowed to do work on its environment by expansion, the heat Q required will be the heat necessary to change the internal energy of the substance from U1 in the first state to U2 in the second state plus the work W done on the environment.
First law of thermodynamics equation
Q = (U2 –U1) + W
Or
Q = ΔU + W
Thus the change in internal energy ΔU =U2 -U1 is defined as Q -W. Since it is the same for all processes concerning the state, the first law of thermodynamics thus can be stated as:
“In any thermodynamic process, when heat Q is added to a system, this energy appears as an increase in the internal energy ΔU stored in the system plus the work W done by the system on its surroundings.”
A bicycle pump provides a good example. When we pump on the handle rapidly,it becomes hot due to mechanical work done on the gas, rising thereby its internal energy.
Human metabolism also provides an example of energy conservation. Human beings and other animals do work. When they walk, run, or move heavy objects, work requires energy. Energy is also needed for growth to make new cells and to replace old cells that have died. Energy transforming processes that occur within an organism or named as metabolism. We can apply the first law of thermodynamics:
1st law of thermodynamics formula
ΔU =Q – W
to an organism of the human body. Work (W) done will result in a decrease in the internal energy of the body. Consequently, the body temperature or in other words internal energy is maintained by the food we eat.
See Also : Second law of thermodynamics
What is an example of the first law of thermodynamics?
A bicycle pump provides a good example. when we pump on the handle rapidly, it becomes hot due to mechanical work done on the gas, raising their by its internal energy. one such simple arrangement is shown in the figure.
It consists of a bicycle pump with a blocked outlet that allows the air temperature to be monitored. when the piston is rapidly pushed, the thermometer shows a temperature rise due to the increase of the internal energy of the air. the push force does work on the air, thereby, increasing its internal energy, which is shown, by the increase in temperature in the air.
First law of thermodynamics and law of conservation of energy
Human metabolism also provides an example of energy conservation. human beings and other animals do work when they walk, run, or move heavy objects. work requires energy. energy is also needed for growth to make new cells and to replace old cells that have died. energy transforming processes that occur within organisms are named as metabolism. we can apply the first law of thermodynamics as :
ΔU = Q-W
to an organism of the human body. work W done will result in a decrease in the internal energy of the body. consequently the body temperature or in other words internal energy is maintained by the food we eat.
Change in internal energy
“A function of thermodynamics coordinates whose final value minus initial value is equal to the value of Q +W in the process is called a change in internal energy.”
The change in internal energy between equilibrium states i and f is given by:
ΔEint =Eint,f – Eint,i
ΔEint=Q +W
The value of internal energy Eint,i depends only on the coordinate of the state ‘i’.Similarly, Eint,f depends only on the state of the system and not at all on the path followed.
Sign convention
- When heat is supplied to the system, it increases the internal energy, so Q is taken as positive(Q > 0)
- Work was done on the system also increases the internal energy, so it is also taken as positive. (W > 0),In this case, the first law of thermodynamics is written as:
ΔEint=Q +W
- When heat is rejected by the system,it decreases the internal energy, so it is taken as negative. (Q <0)
- Work done by the system decreases the internal energy, so it is taken as negative (W <0)
In this case, the first law of thermodynamics is written as:
ΔEint=Q – W
Limitations of 1st law of thermodynamics
The first law of thermodynamics is a general result that is thought to apply to every process in nature which proceeds between equilibrium states.It tells us that energy must be conserved in every process but it does not tell us whether any process that conserves energy can actually occur.
Applications of 1st law of thermodynamics
-
Adiabatic process
“A process in which no heat can enter or leave the system is called an adiabatic process.”In an adiabatic process, there is no transfer of heat across the boundary of the system, so Q=0.According to the first law of thermodynamics:
ΔEint=Q +W
Since
Q = 0 ,SO
ΔEint = W
The work done on the system increases the internal energy.
-
Isothermal process
“A process in which the temperature of the system remains constant is called the Isothermal process.”
Since temperature remains constant in the isothermal process so the internal energy of the gas must also remain constant so:
ΔEint=Q +W
0 = Q + W
⇒ Q =-W
Constant volume process
“The process in which volume of the system remains constant is known as volume process.”
If the volume of a gas remains constant, the work done will be zero, thus W=0
So, according to the first law of thermodynamics:
ΔEint =Q +W
Since W=0,SO
⇒ Q = ΔEint
In this case, all the heat that enters the gas is stored in it as internal energy.
Cyclic process
“It is a series of processes after which system returns to its initial state.”It is a three-step process. It is a cyclic process because it starts and ends at the same point.
U2=U1
U2-U1=0
ΔU=0
From the first law of thermodynamics.
Free expansion
“A process in which gas goes from one side of the container to the other half initially evacuated is called free expansion.”
Watch video about 1st law of thermodynamics:
Zeroth law of thermodynamic equation:
“According to this law, when two bodies have equality of temperature with the third body, then, in turn, they have equality of temperature with each other.”
Watch video about zeroth law
Related topics:
Related Post
Recent Posts
Is energy quantized in classical physics?
No, according to classical wave theory the emission of electromagnetic radiations from the surface is…
Types of laser
Basically, there are four types of laser which includes: Gas Lasers Solid State lasers Liquid…
Ultrasound frequency range
What is ultrasonics? The study and application of mechanical vibrations with frequencies beyond the limits…
Electromagnetic Energy: What are some examples of it?
Electromagnetic energy definition Electromagnetic energy is the amount of energy stored in a region of…
Fundamental units and Derived Units with Examples
The Main Difference between fundamental Units and Base units is that Units that Express base…
Newton’s First law of Motion Examples in Our Daily Life
Newton's first law of motion states that " A body continues its state of rest…
View Comments
fgwdgdvbds