Fundamental units and Derived Units with Examples
 The Main Difference between fundamental Units and Base units is that Units that Express base quantities or fundamental quantities are called Base units or Fundamental Units while Units that describe Derived Quantities are called Derived Units.
The Main Difference between fundamental Units and Base units is that Units that Express base quantities or fundamental quantities are called Base units or Fundamental Units while Units that describe Derived Quantities are called Derived Units.
Units
The fixed and definite quantity taken as the standard of reference with which other quantities of the same kind are measured is defined as a “unit”.
Read Also: Physical quantities
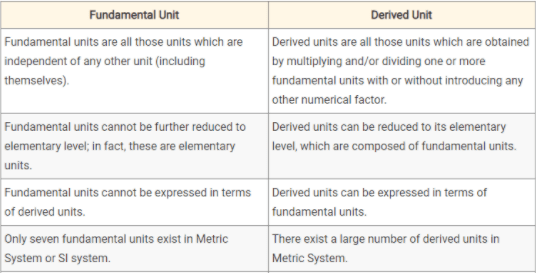
Fundamental Units
The units of fundamental quantities are called “fundamental units”. For example, units of length, mass, and time or those of fundamental units. The table lists all fundamental and supplementary units and their symbols.
| Physical Quantity | SI Unit | Dimensional Symbol | Unit Symbol |
| Length | Meter | L | m |
| Mass | Kilogram | M | kg |
| Time | Second | T | s |
| Temperature | Kelvin | K | k |
| Electric Current | Ampere | A | A |
| Luminous | Candela | I | Cd |
Derived Units
Units of derived physical quantities are called “derived units”. For example, units of velocity, density, force, momentum, and volume.
Initially, three systems of units, namely, CGS, FPS, and MKS based on three fundamental quantities, length, mass, and time, came into existence. In 1970, in world confidence, a consensus evolved, and a standard international system of units.
| Physical Quantity | SI Unit | Dimensional Symbol | Unit Symbol |
| Intensity | |||
| Amount of substance | mole | mol | mol |
| Supplementary units | |||
| Angle | Radian | — | rad |
| Solid angle | stredian | — | str |
Standards of Length
The most common unit is the meter (m), Foot is also used sometimes. In 1889 the standard meter was defined as the distance between two fixed marks engraved on a platinum-iridium bar preserved at a constant temperature of 73.16 K and the constant pressure of 1 bar in the international bureau of weights and measures at Serves near Paris in France. All other meters are calibrated with it to an accuracy of 0.1 ppm.
In 1960 the standard meter was defined in terms of the wavelength of Kr-86 and is called the atomic standard of length.1m is the distance covered by 1650763.73 wavelengths of orange-red light of Kr-86 in the vacuum. An accuracy 1:109 parts can be obtained with it.
In 1983 meter was defined as the length of the path traveled by light in a vacuum in 1/299,792,458th seconds.
Some other important units of the length are
1A = 10-10
1 x-ray unit (1 XU) 10-13m
1 yard = 3 foot = 0.9144 m,
1” (inch) = 2.54 cm
1 astronomical unit (1 AU) = 1.49 x 1011 m.
1 light year (1 ly) = 9.6 x 1015 cm,
1 parsec ( 1 pc) = 3.08 x 1016 m = 3.26 1y
Standard of Mass
Originally, 1 kg mass was defined as the mass of 1 liter (103 cc) of water at 4°C, nowadays standard kg is the mass of platinum-iridium cylinder stored in the special vault in the International Bureau of the standard at Serves (France). The accuracy of this standard is 1 in 108 parts.
To measure atomic masses, the unit amu (or u) is used.
1 u = 1/12th of the mass atom
Or
1 u = 1/12th x kg
= 1.67 x 10-27 kg
In FPS system pound (lb) is the unit of mass, sometimes slug is also used.
1ib = 0.453592737 kg,
1 slug = 32.2 lb = 14.59 kg
In astrophysics, we sometimes come across the Chandrasekhar limit.1 Chandrasekhar limit = 1.4 times mass of sun = 2.8 x 1030 kg. Chandrasekhar showed that if the mass of an object becomes 1.4 times the mass of the sun, under gravitational collapse it turns out to be the white dwarf.
Standards of Time
Initially, it was defined on the basis of the solar or lunar motion.
1 mean solar day = 1 year/ 365.25 days
And 1s = 1 year / 365.25 x 24 x 60 x 60
But because of tidal friction, the length of a day is increasing at a rate of 7 µs per year. Therefore, in 1965 the atomic standard was defined. According to this standard, 1s is the interval of 91192631770 vibrations of the radiation corresponding to the transition between two specific hyperfine levels in 133 Cs (cesium) clocks which will go wrong by 1s in 3000 years. Hydrogen MASER promises a producing error of 1s in 33, 000, 000 years.
(i) Time can never flow back, i.e., negative time does not exist, and (ii) at a given instant of time, a particle cannot be present in more than one position in space.
Related posts:
