Planck’s radiation law derivation

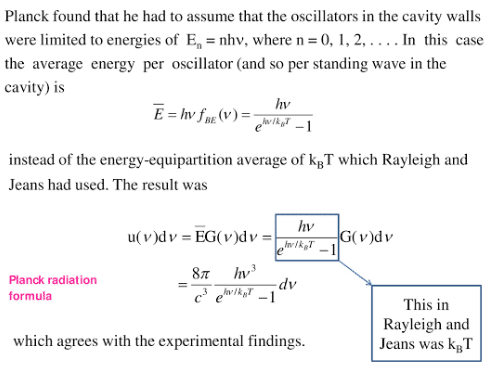
Planck’s quantum theory of radiation
In 1900, Max Planck gave a new concept about the nature of radiation, called the quantum theory of radiation in which Planck assumed the discrete nature of radiation. He assumed the atoms of the cavity emit and absorb radiation in the form of packets of energy, called quanta. The energy of each quantum is directly proportional to frequency.
E ∝ f
E =hf
where h is Planck’s constant.
Max Planck made the following assumptions derive his radiation law:
- The atoms of the cavity behave like tiny harmonic oscillators.
- The oscillators radiate and absorb energy only in the form of packets or bundles of electromagnetic waves.
- An oscillator can emit or absorb any amount of energy which is the integral multiple of “hf ” which is mathematically expressed as:
E=nhf
Where n is an integer.
See Also : Black body radiation
Related Post
Recent Posts
Is energy quantized in classical physics?
No, according to classical wave theory the emission of electromagnetic radiations from the surface is…
Types of laser
Basically, there are four types of laser which includes: Gas Lasers Solid State lasers Liquid…
Ultrasound frequency range
What is ultrasonics? The study and application of mechanical vibrations with frequencies beyond the limits…
Electromagnetic Energy: What are some examples of it?
Electromagnetic energy definition Electromagnetic energy is the amount of energy stored in a region of…
Fundamental units and Derived Units with Examples
The Main Difference between fundamental Units and Base units is that Units that Express base…
Newton’s First law of Motion Examples in Our Daily Life
Newton's first law of motion states that " A body continues its state of rest…