What is SI Unit of density in Physics?
Density and mass are two physical quantities. Mass is the quantity of matter of an object while density is mass per unit volume. They are two different quantities but these quantities have relationships with each other, higher will be the mass higher will be the density. Examples of density are explained below in this article.
What is Density?
“Mass per unit volume of a material is called density.”Density is an important property of any material. A homogeneous material such as ice or iron has the same density throughout. We use (the Greek letter rho) for density.
If one packet contains Styrofoam pellets, for example, and a second packet, similar in size, contains ceramic tiles, it is clear that the second pack will weigh much more than the first pack. Density is a characteristic property that allows identifying different substances.
For example, lead has a density of 11.3 g / cm 3, that of milk is 1.03 g / cm 3 and that of carbon monoxide, a gas very toxic to humans, is just 0 .00125 g / cm 3. The solids tend to have a higher density than liquids and these, in turn, have a higher density than gases.
Density Formula
If a mass m of homogeneous material has volume V, the density is:

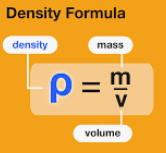
Two objects made of the same material have the same density even though they may have different masses and different volumes. That’s because the ratio of mass to volume is the same for both objects.
See also: Difference between mass and weight
SI Unit of density
The S.I unit of density is the kilogram per cubic meter (1kg/m3). The cgs unit, the gram per cubic centimeter (1g/cm3), is also widely used:
1g/cm3=1000 1kg/m3
The densities of several common substances at ordinary temperatures are given in the table.
The densest material found on earth is the metal osmium (22,500 kg/m3), but its density pales in comparison to the densities of exotic astronomical objects such as white dwarf stars and neutron stars.
Examples of density
Examples of different densities of chemical elements or complex materials, and population densities of cities are given below:
- Naphtha density: 0.70 g / cm 3
- Ice density (at 0 ° C): 0.92 g / cm 3
- Mercury density: 13.6 g / cm 3
- The density of a standard foam rubber mattress: 28 kg / m 3
- Population density of Mexico City (the year 2010): 5862 inhabitants / km²
- The density of Paraná pine wood (dry): 500 kg / m 3
- The density of black carob wood (dry): 800 kg / m 3
- The density of helium (gas with which flying balloons are inflated): 0.000178 g / cm 3
- Uranium density: 18.7 g / cm 3
- The density of regenerating Lenga trees in the Andean-Patagonian forest: 20,000 to 40,000 copies/ha.
How to find the density of a substance?
We can measure the density of a substance in two steps.
- Measure the mass and volume of the substance.
- Divide the mass of the substance by its volume. The result gives the density of the substance.
Density = Mass / Volume
Or
Mass = Density × volume
For example:
Osmium, the densest metal found, has a density of 22.6 g/cm³. The mass of a block of osmium was found to be 113 g. Find its volume.
Sol:
The volume of the block of osmium = Mass /Density
= 113 g/22.6 g/cm³
=5 cm³
Floating and Sinking
Have you ever wondered why some objects float, while others sink?
Objects such as a pebble or a ball bearing sink in water because they have a higher density than water will float. In general, substances with lower densities will float in substances with higher densities.
Specific gravity
The specific gravity of a material is the ratio of its mass/volume to the mass/volume of water at 4.0c0,1000 kg/m3, it is a pure number without units. For example, the specific gravity of aluminum is 2.7.” Specific gravity” is a poor term since it has nothing to do with gravity; relative density would have been better.
The mass/volume of some materials varies from point to point within the material. One example is the material of the human body, which includes low-density fat (about 940 kg/m3) and high-density bone (from 1700 to 2500 kg/m3).
Two others are the earth’s atmosphere (which is denser at greater depths). For these materials equation (1) describes the average density. In general, the density of a material depends on environmental factors such as temperature and pressure.
Measuring mass/volume is an important analytical technique. For example, we can determine the charge condition of a storage battery by measuring the density of its electrolyte, a sulphuric acid solution.
As the battery discharges, the sulfuric acid combines with lead in the battery plates to form insoluble lead sulfate, decreasing the concentration of the solution. The density decreases from about 1.30 3 kg/m3 for a fully charged battery to 1.15 3 kg/m3 for a discharged battery.
Another automotive example is permanent type antifreeze, which is usually a solution of ethylene glycol (3 kg/m3 ) and water. The freezing point of the solution depends on the glycol concentration, which can be performed by using a device called a hydrometer.
Related Topics:
- System international of Units
- Types of physical quantities
- Pascal law
- Archimedes Principle
- Fundamental Units
Related Post
Recent Posts
Is energy quantized in classical physics?
No, according to classical wave theory the emission of electromagnetic radiations from the surface is…
Types of laser
Basically, there are four types of laser which includes: Gas Lasers Solid State lasers Liquid…
Ultrasound frequency range
What is ultrasonics? The study and application of mechanical vibrations with frequencies beyond the limits…
Electromagnetic Energy: What are some examples of it?
Electromagnetic energy definition Electromagnetic energy is the amount of energy stored in a region of…
Fundamental units and Derived Units with Examples
The Main Difference between fundamental Units and Base units is that Units that Express base…
Newton’s First law of Motion Examples in Our Daily Life
Newton's first law of motion states that " A body continues its state of rest…
View Comments
Thank very much for helping me.
This info is so great thanks!