Types of electric charge and their properties

Electric charge is defined as: “An electrical property of matter that exists because of access or a deficiency of electrons.”There are two types of electric charges, positive charges, and negative charges. Like charges repel each other while unlike charges attract each other. SI Unit of Electric charge is Coulomb.
Electric Charge Formula
Its formula is given as:
Q =I × t
Where Q is the total charge, I is the amount of current and t is the time.
Electric charge examples
If you walk across a carpet in dry weather, you can draw a spark by touching a metal doorknob. On a grander scale, lightning is familiar to everyone. Such phenomena suggest the vast amount of electric charge that is stored in the familiar objects that surround us.
The electrical neutrality of most objects in our visible and tangible world conceals their content of enormous amounts of positive and negative electric charge that largely cancel each other in their external effects. Only when this electrical balance is disturbed does nature reveal to us the effects of uncompensated positive or negative charge. When we say that a body is “charged” we mean that it has a charge imbalance, even though the net charge generally represents only a tiny fraction of the total positive or negative charge contained in the body.
Charged bodies exert forces on each other. To show this, let us charge a glass rod by rubbing it with silk. The process of rubbing transfers a tiny amount of charge from one body to the other, thus slightly upsetting the electrical neutrality of each.
See Also : Coulomb’s law
How charges are produced?
If we run a plastic comb through our hair and then bring it near small pieces of paper, the comb attracts them. Similarly, amber when rubbed with silk, attracts small pieces of paper. This property of attraction or repulsion between substances is due to the electric charges they acquire rubbing.
We can produce an electric charge by rubbing a neutral body with another neutral body. The following activities show that we can produce two types of electric charges through the process of rubbing.
Take a plastic rod. Rub it with fur and suspend it horizontally by silk thread in the above Figure. Now take another plastic rod and rub it with fur and bring it near to the suspended rod. We will observe that both the rods will repel each other. It means during the rubbing both the rods were charged.
Now take a glass rod and rub it with silk and suspend it horizontally. When we bring the plastic rod rubbed with fur near to the suspended glass rod, we observe that both the rod each other.
Rods are unlike and their attraction implies that charges on two rods are not of the same kind but of opposite nature.
These opposite charges are conventionally called a positive charge and a negative charge. During the process of rubbing a negative charge is transferred from one object to another object.
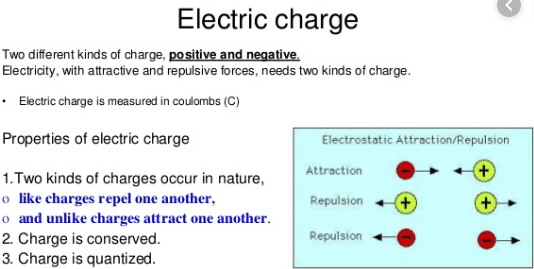
Properties of electrical charge
- The charge is a basic property of a material body due to which attracts or repels another object.
- Friction produces two different types of charges on different materials (such as glass or plastic).
- Like charges always repel each other.
- Unlike charges always attract each other.
- Repulsion is the sure test of charge on a body.
What are the two types of electric charge?
Basically, there are two types of charge, which are given below:
Positive Charge
“When a material losses electrons then the number of protons increase in the material, there is a net positive electrical charge .”
The positive and negative labels for electric charges are due to Benjamin Franklin (1706-1790) who, among many other accomplishments, was a scientist of international reputation. It has even been said that Franklin’s triumphs in diplomacy in France during the American War of independence may have been made possible because he was so highly regarded as a scientist.
Electrical forces between charged bodies have many industrial applications, among them, being electrostatic paint spraying and powder coating, fly ash precipitation, non-impact inkjet printing, and photocopying. For example, shows a tiny carrier bead in a photocopying machine, covered with particles of black powder called toner, that stick to the carrier bead by electrostatic forces.
These negatively charged toner particles are eventually attracted from their carrier beads to a positive charge latent image of the document to be copied, which is formed on a routing drum. A charged sheet of paper then attracts the toner particles from the drum to itself, after which they are heat fused in place to make the final copy.
Negative Charge
“When a material gains electrons then the number of electrons increase in the material, there is a net negative electrical charge.”
The electron has a negative charge, while the proton has a positive charge. in a material number of protons, electrons are equal in numbers. Electrical charge is a, which is a fundamental characteristic of electrons and protons, is symbolized by Q.
Static electricity is the presence of net positive and negative charges in a material. Everyone has experienced the effects of static electricity from time to time, for example when attempting to touch a metal surface or another person or when clothes in the dryer cling together.
Like charges repel each other and unlike charges attract each other, A force acts between charges, as evidenced by the attraction or repulsion. This force, called an electric field, consists of invisible lines of force.
How electroscopes detect charge?
The gold leaf electroscope is a sensitive instrument for detecting charges. It consists of a brass rod with a brass disk at the top and two thin leaves of gold foil hanging at the bottom in Fig. The rod passes through an insulator that keeps the rod in place. Charges can move freely from the disk to the leaves through the rod. A thin aluminum foil is attached to the lower portion of the inside of the jar. Usually, the aluminum foil is grounded by connecting a copper wire. This protects the leaves from external electrical disturbances.
Detecting The Presence of Charge:
In order to detect the presence of charge on anybody, bring the body near the disk of an uncharged electroscope. If the body neutral there will be no deflection of the leaves in above Fig:
But if the body is positively or negatively charged, the leaves of the electroscope diverge. For example, if the body is negatively charged then due to electrostatic induction, a positively charge will appear on the disk while a negative charge will appear on the leave-in above Fig:
The leaves of the electroscope repel each other and diverge because each leave gets a similar charge. The divergence of leaves will depend on the amount of charge.
Charging the Electroscope by Electrostatic Induction:
Electroscope can be charged by the process of electrostatic induction. In order to produce a positive charge on the electroscope, bring a negatively charged body near the disk of the electroscope in above Fig:
The positive charge will appear on the disk of the electroscope while the negative charges will shift to the leaves. Now connect the disk of electroscope to the earthed aluminum foil by a conducting wire in above Fig:
The charge of the leaves will flow to the Earth through the wire. Now if we first break the Earth connection and then remove the rod, the electroscope will be left with a positive charge in above Fig:
Similarly, an electroscope can be charged negatively with the help of a positively charged rod.
Electroscope can also be changed by the process of conduction. Touch a negatively charged rod with the disk of a neutral electroscope. A negative charge from the rod will transfer to the electroscope and will cause it leaves to diverge.
Detecting the Type of Charge:
For the detection of the type of charge on a body, the electroscope is first charged either positively. Suppose the electroscope is positively charged as explain before and are shown in above Fig:
Now in order to detect the type of charge on a body, bring the charged body near the disk of the positively charged electroscope. If the divergence of the leaves increases, the body carries positively charges are shown in above Fig:
On the other hand, if the divergence decreases, the body has negative charge are sown in above Fig:
Identifying Conductors and Insulators:
An electroscope can also be used to distinguish between insulators and conductors. Touch the disk of a charged electroscope with the material under test. If the leaves collapse from their diverged position, the body would be a good conductor. If there is no change in the divergence of the leaves, it will show that the body under test in an insulator.
What is the unit of electrical charge?
Electric charge(Q) is measured in Coulombs, symbolized by C.
To learn more about charges watch the video:
The charge is conserved:
- In an isolated system, a charge can neither be created nor destroyed.
- The total charge in the universe is constant.
- The charge can be created or destroyed but in equal and opposite pairs, for instance, a γ-ray can split be an electron and a positron,i.e,
If Eγ (≥ 1.02 MeV)
γ→ e- + e+
This process is called “pair production”.The electron and positron can combine to form γ-ray again. Such a process is termed as pair annihilation.
e- + e+ → γ(Eγ =1.02 MeV)
The charge is Quantised:
Charge on a body can be an integral multiple of electronic charge.i.e.Q=± n e. If a body gains electrons, it is said to be negatively charged and if it loses electrons, it is said to be positively charged. Though there are particles called “quarks” which may have charges e/3 or 2e/3.Since these are generated during the disintegration of the nucleus (neutron, proton, and so on ) these cannot be transferred. The charge on an electron is 1.6× 10-19C.
Related topics:
- Ohm’s law formula
- Coulomb’s law formula
- Gauss’s law applications
- Difference between conductors and insulators
Suggested Physics Links:
Let’s Dive in:
Suggested Referal Links:
https://en.wikipedia.org/wiki/Electric_charge
https://www.britannica.com/science/electric-charge
https://byjus.com/physics/electric-charge/
Recent Posts
Is energy quantized in classical physics?
No, according to classical wave theory the emission of electromagnetic radiations from the surface is…
Types of laser
Basically, there are four types of laser which includes: Gas Lasers Solid State lasers Liquid…
Ultrasound frequency range
What is ultrasonics? The study and application of mechanical vibrations with frequencies beyond the limits…
Electromagnetic Energy: What are some examples of it?
Electromagnetic energy definition Electromagnetic energy is the amount of energy stored in a region of…
Fundamental units and Derived Units with Examples
The Main Difference between fundamental Units and Base units is that Units that Express base…
Newton’s First law of Motion Examples in Our Daily Life
Newton's first law of motion states that " A body continues its state of rest…
View Comments
Great work! please keep it up.
E-BOOK