Difference between Evaporation and boiling with examples

What does evaporation mean?
“Evaporation is the changing of a liquid into vapours (gaseous state) from the surface of the liquid without heating it.”Take some water in a dish.
The water in the dish will disappear after some time. It is because the molecules of water are in constant motion and possess kinetic energy. Fast-moving molecules escape out from the surface of the water and go into the atmosphere. This is called evaporation.
Unlike boiling, evaporation takes place at all temperature but only from the surface of a liquid. The process of boiling takes at a certain fixed temperature which is the boiling point of that liquid. At boiling point, a liquid is changing into vapours not only from the surface but also within the liquid. These vapours come out of the boiling liquid as bubbles that break down on reaching the surface.
How evaporation causes cooling?
Evaporation causes cooling because water on your body is evaporating. Evaporation needs heat and in this process for example, heat comes from our body. Since our body loses heat when the water evaporates, we feel cold.
What is Boiling?
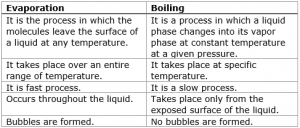
Difference between evaporation and boiling in tabular form
| Evaporation | Boiling |
| It occurs at any temperature. | It occurs at a fixed temperature. |
| It is a slow process. | It is a fast or quick process. |
| It takes place only on the surface of the liquid. | It takes place in the liquid. |
| In this process, no bubbles are formed in liquid. | In this process, bubbles are formed in liquid. |
| The temperature of this process may change. | The temperature remains constant in the boiling process. |
| Heat supplied by the surroundings. | Heat supplied by an energy source. |
Related Topics:
Related Post
Recent Posts
Is energy quantized in classical physics?
No, according to classical wave theory the emission of electromagnetic radiations from the surface is…
Types of laser
Basically, there are four types of laser which includes: Gas Lasers Solid State lasers Liquid…
Ultrasound frequency range
What is ultrasonics? The study and application of mechanical vibrations with frequencies beyond the limits…
Electromagnetic Energy: What are some examples of it?
Electromagnetic energy definition Electromagnetic energy is the amount of energy stored in a region of…
Fundamental units and Derived Units with Examples
The Main Difference between fundamental Units and Base units is that Units that Express base…
Newton’s First law of Motion Examples in Our Daily Life
Newton's first law of motion states that " A body continues its state of rest…
View Comments
a