Difference between Evaporation and boiling with examples
What does evaporation mean?
“Evaporation is the changing of a liquid into vapours (gaseous state) from the surface of the liquid without heating it.”Take some water in a dish.
The water in the dish will disappear after some time. It is because the molecules of water are in constant motion and possess kinetic energy. Fast-moving molecules escape out from the surface of the water and go into the atmosphere. This is called evaporation.
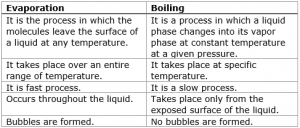
Unlike boiling, evaporation takes place at all temperature but only from the surface of a liquid. The process of boiling takes at a certain fixed temperature which is the boiling point of that liquid. At boiling point, a liquid is changing into vapours not only from the surface but also within the liquid. These vapours come out of the boiling liquid as bubbles that break down on reaching the surface.
How evaporation causes cooling?
Evaporation causes cooling because water on your body is evaporating. Evaporation needs heat and in this process for example, heat comes from our body. Since our body loses heat when the water evaporates, we feel cold.
What is Boiling?
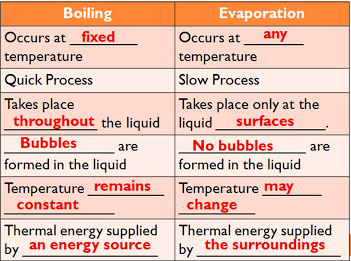
Difference between evaporation and boiling in tabular form
| Evaporation | Boiling |
| It occurs at any temperature. | It occurs at a fixed temperature. |
| It is a slow process. | It is a fast or quick process. |
| It takes place only on the surface of the liquid. | It takes place in the liquid. |
| In this process, no bubbles are formed in liquid. | In this process, bubbles are formed in liquid. |
| The temperature of this process may change. | The temperature remains constant in the boiling process. |
| Heat supplied by the surroundings. | Heat supplied by an energy source. |
Related Topics:
a