
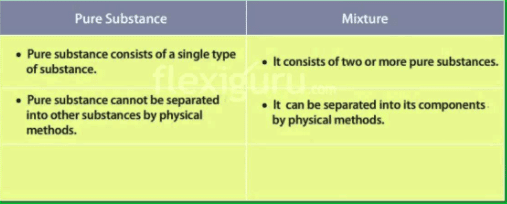
The Difference between Pure Substance and Mixture is given here. For chemistry, a pure substance is one whose chemical composition is homogeneous while a mixture is the union of various substances with different properties that do not react chemically.
keep reading…
What is Pure substance?
It is a substance that has an invariable and homogeneous chemical composition. It can appear in different phases: solid, liquid, and gas depending on the pressure and temperature to which it is subjected.
It can also occur in two or three phases at the same time. Although it sounds surprising, there are several situations in which two phases of a substance can coexist in equilibrium.
What is a Mixture?
Mixtures are materials formed by the union of two or more components that have not been chemically united. This means that in a mixture there are no chemical reactions and each of its components maintains its properties and its identity.
Mixtures do not have chemical reactions, but they can be reactive, that is, their components can react if they are subjected to certain environmental conditions.
The mixture is considered to be the physical combination of two or more substances that maintain their identities.
A mixture is the result of the mechanical mixing of chemical substances without these forming bonds or reactions.
Mixtures can be separated into their components by physical or thermal processes.
Mixtures can be classified into:
- Homogeneous: They can be classified into coarse mixtures, suspensions, colloidal dispersion, and chemical suspension.
- Heterogeneous.
suggested video: Pure Substance vs Mixture
Difference between Pure Substance and Mixture
- Pure substances are those that have a uniform or fixed composition. It has chemical and physical properties and characteristic properties.
- A mixture is the union of different molecules that do not react chemically with each other. They can be formed by sets of compounds or elements.
- The mixtures do not have specific melting or boiling points, that is, they do not have characteristic properties. They can react if they are subjected to certain conditions.
Related Post
Recent Posts
Is energy quantized in classical physics?
No, according to classical wave theory the emission of electromagnetic radiations from the surface is…
Types of laser
Basically, there are four types of laser which includes: Gas Lasers Solid State lasers Liquid…
Ultrasound frequency range
What is ultrasonics? The study and application of mechanical vibrations with frequencies beyond the limits…
Electromagnetic Energy: What are some examples of it?
Electromagnetic energy definition Electromagnetic energy is the amount of energy stored in a region of…
Fundamental units and Derived Units with Examples
The Main Difference between fundamental Units and Base units is that Units that Express base…
Newton’s First law of Motion Examples in Our Daily Life
Newton's first law of motion states that " A body continues its state of rest…
