Examples of Fats
Fats are organic substances, that is, their molecules are composed mainly of carbon, oxygen, and hydrogen atoms. They are the classes of lipids that contain fatty acids. For example peanuts, tuna, margarine, and butter.

Here we refer to all lipids that contain fatty acids. However, lipids that are in a solid state at room temperature and are of animal origin are usually called “fat”, while lipids that are in a liquid state at room temperature, and that in Most are of plant origin.
It is a very heterogeneous group, but all fats have characteristics in common:
- Insoluble in water. That is why water and substances such as oil do not mix.
- Soluble in organic solvents. Fats can dissolve in benzene, ether, chloroform, alcohol, and other organic substances. For this reason, when cleaning kitchens, alcohol is used to remove grease from surfaces.
- They have a lower density than water. Tiny bubbles of oil float in liquids like soups and broths.
- Slippery and shiny. Much of the lipids are slippery to the touch and shiny on their surface.
Read Also: Good fats Vs bad fats
Functions of fats
One of the main functions of fat is to store energy. While a gram of carbohydrate or protein has 4 kilocalories, a gram of fat has 9 kilocalories. Therefore, fat is the ideal material to store energy.
However, fats fulfill other functions, such as creating tissues that insulate from the cold, protect organs, participate in the absorption of vitamins and the synthesis of hormones, form cell membranes and wrap nervous tissue.
What are essential fatty acids?
Substances that the human body needs for its proper functioning and cannot produce on its own are called “ essential ”. That’s why you need to get them from food.
In the case of fats, there are essential fatty acids that are essential for the human body, such as linoleic, arachidonic, and linolenic. These fatty acids are obtained from vegetable oils.
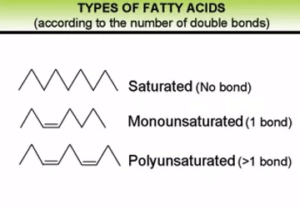
What is saturation?
Fats are usually made up of a glycerin molecule and one, two, or three fatty acids. When a compound is saturated, all of its atoms are held together by a single bond and there is no chance for any other atoms to join. This is what is meant by saturation: the maximum number of atoms attached to the molecule has been reached. Due to their molecular structure, saturated fats are solid at room temperature.
Conversely, if a compound is unsaturated, the atoms are linked by more than one bond. In these cases, double or triple bonds are observed. Due to their molecular structure, unsaturated fats are liquid at room temperature.
What is trans fat?
When a fatty acid is unsaturated, its double or triple bonds can be broken through hydrogen atoms. Let’s imagine the links as joined hands. If two carbon atoms are double-bonded to each other (their two hands are joined together) a hydrogen atom can be added to each: each can “shake hands” with the new hydrogen atom, and so on. they will still be attached to the other carbon atom by the remaining bond.
This process is called hydrogenation and it is the process by which unsaturated fats become saturated. This process occurs naturally only in the milk and body fat of cows and other ruminants.
However, it is widely used in industry, since it allows oils to solidify, giving foods a special texture, in addition to being less vulnerable to rancidity.
dangers of saturated fats
Although fats are essential for the functioning of the body, not all of them are beneficial.
Saturated fats favor the accumulation of cholesterol (low-density cholesterol or LDL) in the walls of the arteries. This accumulation hinders blood circulation, affecting all organs and tissues, including the brain.
Trans fats have all the risks of saturated fats, but they also lower the so-called “good cholesterol”: high-density cholesterol (HDL). HDL cholesterol collaborates with the maintenance of the inner walls of the veins, preventing atherosclerosis, heart attacks, and strokes. The consumption of trans fats decreases this protection.
In addition, excessive consumption of any type of fat favors the excessive accumulation of adipose tissue (fat) in the body. Although we all need the energy reserve that adipose tissue represents, its excess causes diseases such as obesity. Obesity is not only an aesthetic problem but is associated with circulatory, metabolic, joint, respiratory, and digestive diseases and can even cause psychiatric disorders.
Infrequent consumption of saturated fat does not necessarily cause problems unless there is a natural predisposition of the body for cardiovascular problems. In addition, some of the harmful effects of measured consumption of saturated fat can be counteracted by lifestyle, such as exercise.
examples of unsaturated fats
| Olive oil | Peanut |
| Tuna | olives |
| Salmon | sardines |
| Avocado (avocado) | Corn oil |
| Walnuts | Sunflower oil |
| almonds | Soy oil |
Read Also: Macronutrients and micronutrients
Examples of saturated fats
| Cow meat | cheeses |
| Pancetta (bacon) | chicken skin |
| offal (viscera) | Cream ice cream |
| Butter | Pork Meat |
| Milk | Mutton |
Examples of trans fats
- Margarine
- Some cookies
- Fried in reused oil or that exceeds 180 degrees
- Most fast food
- Bills
- snacks
- Some pre-cooked dishes
- Cereal bars
- Industrial pastry products (muffins, sponge cakes, etc.)
