Difference between element and compound
In chemistry, A chemical element is a product made up of atoms of the same class, it is the simplest form of matter while a compound is a substance formed by the union of two or more elements. keep reading…
What is an Element?
A chemical element is a matter made up of equal atoms. It is the simplest form of matter and its atomic characteristics allow you to classify it by atomic number on the periodic table. The elements are represented on the periodic table. It is an atom with unique physical characteristics, it is a substance that cannot be decomposed by a chemical reaction into simpler ones. If there are two atoms of the same element with different characteristics such as its mass number, then they will be known as its isotopes.
What is a Compound?
It is a substance formed by the union of two or more different elements of the periodic table. Compounds are represented by a chemical formula. They have intrinsic characteristics and properties such as a constant composition and their components are always in constant proportions. A compound is made up of molecules or ions with stable bonds. They are classified into two groups: Inorganic compounds
- Basic oxides.
- Acidic oxides.
- Hydrides
- Hydracids.
- Hydroxides
- Oxacids.
- Oxisales.
Organic compounds:
- Aliphatic compounds.
- Aromatic compounds.
- Heterocyclic compounds.
- Organometallic compounds.
- Polymers
suggested video: element and compound
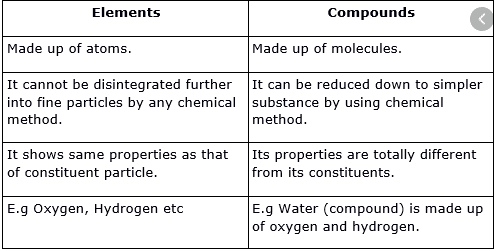
Difference between element and compound in points
- Elements are those substances that represent the simplest form of matter.
- An item cannot be divided or separated by physical processes. Decantation, distillation, or filtration.
- Elements are pure substances made up of a single type of atom.
- A compound is a pure substance made up of several elements. It has a unique structure.
- Compounds can be separated into elements by various processes.
- Compounds seek to be more stable than elements.