To form the molecules of chemical compounds, the atoms of different substances or elements must combine with each other in a stable way. This can happen in various ways by virtue of the structural characteristics that every atom has (consisting of a positively charged nucleus surrounded by a cloud of electrons).
The electrons have a negative charge and remain close to the nucleus because the electromagnetic force of the protons attracts them. The closer an electron is to the nucleus, the greater the energy required to get it released.
But not all elements are the same: some have a tendency to lose the outermost electrons of the cloud (elements with low ionization energy), while others tend to capture them (elements with high electron affinity). This happens because according to the Lewis octet rule, stability is associated with the presence of 8 electrons in the outermost shell or orbital (region of space where an electron is most likely to be found around the atom), at least in most cases. the cases.
Due to this, to form the different chemical compounds, the neutral atoms give up, accept or share the electrons of their last electronic shell, always trying to keep 8 electrons in it, although there are always exceptions, such as the case of hydrogen that can only have 2. electrons.
See also: Metallic bond
What are ionic bonds?
So, as neutral atoms can gain or lose electrons, ions of opposite charges can be formed. The electrostatic attraction between ions of opposite charge causes the ions to join together and form chemical compounds, in which one of the elements gave up electrons and the other received them. For this to happen and an ionic bond to form, there must be a difference or delta of electronegativity between the elements involved of at least 1.7.
Ionic bonding usually occurs between a metallic and a nonmetallic compound: the metal atom gives up one or more electrons, thereby forming positively charged ions (cations), and the nonmetal gains them and becomes the negatively charged particle (anion). The alkali metals and alkaline earth metals are the elements that have the greatest tendency to form cations, and the halogen elements and oxygen are the ones that usually constitute the anions.
In general, the compounds that are formed by ionic bonds are crystalline solids at room temperature, insoluble in water, and of high melting point, in case the attractions between their ions are strong. On the other hand, when the attraction between their ions is weaker, they have lower melting points and are soluble in water.
In solution they are very good conductors of electricity since they are strong electrolytes, that is, they ionize easily forming anions and cations that can carry electrical charges. On the other hand, the lattice energy of an ionic solid is what marks the force of attraction between the ions of that solid.
It is important to clarify that there is neither a completely ionic bond nor a completely covalent bond (which occurs between two atoms that share the electrons of their last energy level or shell). Actually, both types of links have a percentage of each. Some scientists consider ionic bonding to be an exaggeration of covalent bonding.
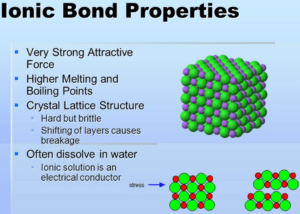
Ionic Bond Properties
Common Characteristics of Ionic Bond are:
- When ionic bonds are kept at normal temperatures, they can remain solid.
- It has a crystalline or translucent structure.
- Their melting and boiling points are both very high.
- They are bonds formed by the interaction of metals from groups I and II with non-metals from groups VI and VII.
- They are highly powerful and fully reliant on ions.
- They are water and other aqueous solution solubilized. This is due to the fact that they have an electric dipole that can separate the ions.
- When in an aqueous solution, they are good conductors of electricity.
- They are incapable of conducting any form of electricity while they are in the solid-state.
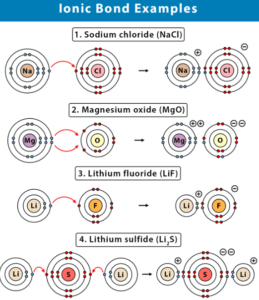
Ionic bond Examples
- Magnesium Oxide (MgO)
- Copper sulfate (II) (CuSO 4 )
- Potassium iodide (KI)
- Zinc hydroxide (Zn(OH) 2 )
- Sodium chloride (NaCl)
- Silver nitrate (AgNO 3 )
- Lithium fluoride (LiF)
- Magnesium chloride (MgCl 2 )
- Potassium hydroxide (KOH)
- Calcium nitrate (Ca(NO 3 ) 2 )
- Potassium dichromate (K 2 Cr 2 O 7 )
- Disodium phosphate (Na 2 HPO 4 )
- Iron (III) sulfide (Fe 2 S 3 )
- Potassium bromide (KBr)
- Calcium carbonate (CaCO 3 )
- Sodium hypochlorite (NaClO)
- Potassium sulfate (K 2 SO 4 )
- Manganese(II) chloride (MnCl 2 )
- Calcium phosphate (Ca 3 (PO 4 ) 2 )