Difference between reversible and irreversible process in thermodynamics
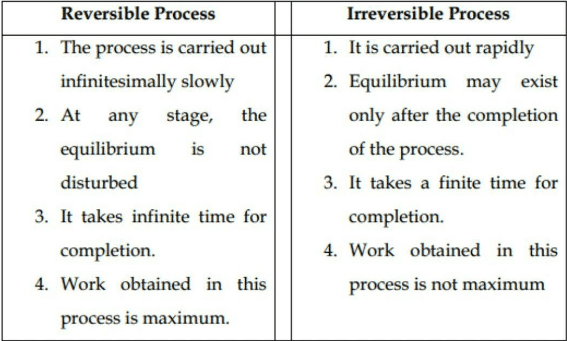 The basic difference between reversible and irreversible processes is that in the reversible process the system remains in thermodynamic equilibrium, while in the irreversible process the system does not remain in thermodynamic equilibrium.
The basic difference between reversible and irreversible processes is that in the reversible process the system remains in thermodynamic equilibrium, while in the irreversible process the system does not remain in thermodynamic equilibrium.
Reversible process in thermodynamics
“A process which can be retracted in exactly reverse order without producing any change in the surroundings is called the reversible process”.
In the reverse process, the system passes through the same stages as in the direct process but thermal and mechanical effects at each stage are exactly reverse. If heat is absorbed in the direct process, it will be given out in the reverse process. Similarly, if work is done by the system in the direct process, work will be done on the system in the reverse process. Hence the system will restore to the original state.
Conditions for the reversible process
There are two important conditions for the reversible process to occur.
- Firstly, the process should occur in infinitesimally small time.
- Secondly. all the initial and final states should be in equilibrium with each other.
Reversibility in thermodynamics
The phenomenon of undergoing reversible change is also called reversibility. In actual practice the reversible process never occurs, thus it is an ideal or hypothetical process.
Reversible process example
Although no actual change is completely reversible by the process of liquefaction and evaporation of a system performed slowly are practically reversible. Similarly slow compression of the gas in a cylinder is a reversible process as gas can be expanded slowly by decreasing the weight on the piston to reverse the operation.
Irreversible process in thermodynamics
The process which cannot be retracted in the opposite order by reversing the controlling factors is called an irreversible process.
Most of the processes in nature are irreversible. During an irreversible change, the system is not equilibrium at all instances of time. The irreversible process consists of non-equilibrium states which cannot be represented on a P-V diagram.
All changes which occur suddenly or which involve friction or dissipation of energy through conduction, convection, or radiation are irreversible.
See Also related topics on our Page: Thermodynamics
Some important points about the irreversible process
- In the irreversible process, the initial stage of the system and surroundings cannot be restored from the final stage.
- During the irreversible process of the various states of the system of the path of change from the initial stage to the final state are not in equilibrium with each other.
- During the irreversible process, the entropy of the system increases decisively and it cannot be reduced back to its initial value.
- The phenomena of a system undergoing irreversible processes are called irreversibility.
Irreversible process examples
- The conduction of heat from a hot body to a cold body.
- Production of heat by the friction
- Producing of heat by the passing of current through an electrical resistance
- Transfer of heat by radiation
- An explosion
- Inelastic deformation
- Magnetization or polarization with a hysteresis
- Spontaneous chemical reactions
- Spontaneous mixing of the matter of varying states
Related topics:
