Types of Natural Radioactivity
What is Natural-radioactivity?
All the elements beyond hydrogen and helium were made in nuclear reactions in the interiors of stars or in explosive supernovas. Both radioactive and stable nuclides are created in these processes. The solar system is composed of nuclides that were formed about 4.5×109 years ago. (How this is determined is discussed later in this section).
Most of the radioactive nuclides that were formed at that time have half-lives that are far shorter than a billion years, and so they have long since decayed to stable nuclides through alpha or beta emission. A few of the original radioactive nuclides, however, have half-lives that are not short in comparison with the age of the solar system. The decay of these nuclides can still be observed, and these decay form part of the background of the natural radioactivity in our environment.
Some of these radioactive species are part of decay chains that start with heavy nuclides, such as 232Th (t 1/2 = 1.4×10 10 y) or 238 U (t 1/2 = 4.5 ×10 9 y). These nuclides decay through a sequence of alpha and beta decays, eventually reaching stable products (respectively, 208 Pb and 206 Pb). The intermediate nuclei in these decay chains have much shorter half-lives; the rate at which the original nuclide disappears and is replaced with the stable end product is determined by the longest-lived member of the chain.
These decay processes have presumably been going on since the solar system was formed, and so(so we discuss later) the relative amount of the initial nuclide and stable decay products present in the material can give a measure of the age of the material. These decay are also thought to contribute to the internal heating of the planets.
Normally, the products of this decay remain in place in the rocks or minerals containing the parent nuclide. However, one of the intermediate substances produced in these decay chains, radon, is a gas. Natural decays that occur near the surface of the Earth(and in building materials, such as concrete) release radioactive radon gas and are currently the subject of active research.
Radon gas can also be released from the feature of the rocks beneath the surface; therefore the detection of radon gas has been used as a way of predicting earthquakes.
In addition to the heavy elements, other long-lived radioactive nuclides are present in natural substances. Some of these are listed in Table.
Other radioactive nuclides are continually produced by natural processes, generally in the Earth’s atmosphere by reactions of molecules of the air with cosmic rays (high energy protons from space). Notable among these is 14 C (t 1/2 = 5730 y), which has important applications in the radioactive dating of organic material.
Radioactive Dating
Suppose we have an initial radio-nuclide I that decays to a final product F with a known half-life t 1/2 . As a particular time t=0, we start with N0 initial nuclei and non of the final product nuclei. At a later time t, we find N1 of the product nuclei have appeared . The initial nuclei decay according to:
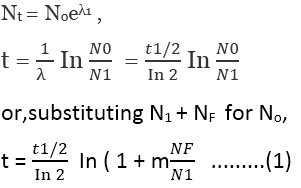
This is a measurement of the present ratio of the product and original nuclei can determine the age of the sample.
This calculation has been based on the assumption that may not always be valid, but there are techniques for radioactive dating that can correct for the presence of these original product nuclei.
This method can be used to determine the time since the formation of the solar system; the example includes the ratio of 238 U to 206 Pb, 87 Rb to 87 Sr, and 40 K to 40 Ar. Terrestrial rocks, moon rocks, and meteorites analyzed by these methods all seem to have common ages of around 4.5×109 y, which we take to be the age of the solar system.
The radioactive isotope 14 C is present in the atmosphere; about 1 carbon atom 1012 is radioactive 14 C. Each gram of carbon has an activity of about 12 decays per minute due to 14 C. Living organisms can absorb this activity by aspiration of CO2 or by eating plants that have done so.
When the organism dies, it stops absorbing 14 C, and the 14C present at its death begins to decay. By measuring the decay rate of 14 C, we can determine the age of the sample.
For example, if we examine a sample and it shows 6 decays per minute per gram of carbon, we have known that the original activity has been reduced by half, and the sample must be half-life (5730 y) old.
This website is really cool. I have bookmarked it.
Do you allow guest post on your blog ? I can write high quality posts
for you. Let me know.