Difference between nuclear fission and nuclear fusion
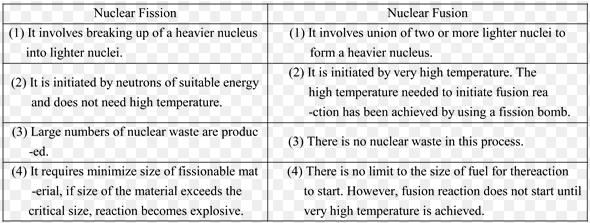
Nuclear fission and Nuclear Fusion are the Types of Nuclear Reactions. The basic difference between nuclear fission and fusion is that in fission reaction, a heavy nucleus splits into two daughter nuclei with the emission of energy, while in nuclear fusion two lighter nuclei combine to form of heavy nuclei.
what is Nuclear Fission?
“Such a reaction in which a heavy nucleus like that of uranium splits up into two nuclei of equal size along with the emission of energy during the reaction is called fission reaction”
Otto Hahn and Fritz Strassman of Germany while working upon the nuclear reactions made a startling discovery. They observed that when slow-moving neutrons are bombarded on ![]() , then as a result of a nuclear reaction
, then as a result of a nuclear reaction ![]() ,
, ![]() an and average of three neutrons are obtained. It may be remembered that the mas of both Krypton and barium is less than that the mass of uranium.
an and average of three neutrons are obtained. It may be remembered that the mas of both Krypton and barium is less than that the mass of uranium.
This nuclear reaction was different from other to studied other nuclear reactions in two ways. First, as a result of the breakage of the uranium nucleus two nuclei of almost equal size are obtained, whereas in the other nuclear reactions the difference between the masses of the reactants and the products was not large. Secondly, a very large amount of energy is given out in this reaction. Fission reaction can be represented by the equation:
![]()
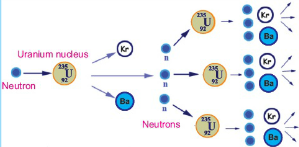
Here Q is the energy give out in this reaction, By comparing the total energy on the left side of the equation with total energy on the right side, we find that in the fission of one uranium nucleus about 200 MeV energy is given out. It may be kept in mind that three is no difference between the sum of the mass and the charge number on both sides of the equation.
Fission reaction can be easily explained with the help of a graph. This graph shows that the binding energy per nucleon is greatest for the middle elements of the periodic table and this bending energy per nucleon is a little less for the light or very heavy elements i.e., then nucleons in the light or very heavy elements are not so rigidly bound.
For example, the binding energy per nucleon for uranium is about 7.7 MeV and the products of the fission reaction of uranium, namely barium and krypton, have a total mass less than the mass of uranium equal to 8.5 -7.6 = 0.9 MeV per nucleon. Thus when a uranium nucleus breaks up, as a result of fission reaction, into barium and krypton, then energy at the rate of 0.9 MeV per nucleon is given out. This means that an energy 235 × 0.9 = 211.5 MeV is given out in the fission of one uranium nucleus.
The fission process of uranium does not always produce the same fragments (Ba, Kr). In fact, any of the two nuclei present the upper Horizontal part of binding energy that could be produced. Two possible fission reactions of uranium are given below as an example:
![]()
Hence in the uranium fission reaction, several products may be produced. All of these products (fragments) are radioactive. Fission reaction is not confined to uranium along it is possible in many other heavy elements. However, it not been observed that fission takes place very easily with the slow neutrons in uranium-235 and plutonium-239, and mostly these two are used for fission purposes.
Fission Chain Reaction
We have observed that during fission reaction a nucleus of uranium-235 absorbs a neutron and breaks into two nuclei of almost equal masses besides emitting two or three neutrons. By properly using these neutrons fission reaction can be produced in more uranium atoms such that a fission reaction can continuously maintain itself. The process is called a fission chain reaction.
Suppose that we have a definite amount of ![]() and a slow neutron originating from any source produces fission reaction in one atom of uranium. Out of this reaction, about three neutrons are emitted. If conditions are appropriate these neutrons produce fission in some more atoms of uranium. In this way, this process rapidly proceeds and in an infinitesimally small time a large amount of energy along with huge explosion produces, it is a representation of fission chain reaction
and a slow neutron originating from any source produces fission reaction in one atom of uranium. Out of this reaction, about three neutrons are emitted. If conditions are appropriate these neutrons produce fission in some more atoms of uranium. In this way, this process rapidly proceeds and in an infinitesimally small time a large amount of energy along with huge explosion produces, it is a representation of fission chain reaction
It is possible to produce such conditions in which only or neutron, out of all the neutrons created in one fission reaction becomes the cause of further fission reaction. The other neutrons either escape out or are absorbed in any other medium except uranium. In this case, the fission chain reaction proceeds with its initial speed. To understand these conditions carefully look at it.
The resulting neutrons scatter in the air and so the cannot produce any fission chain reaction. Some favorable conditions for a chain reaction. Some of the neutrons produced in the first fission reaction produce only one more fission reaction but here also no chain reaction is produced. If the sphere sufficiently big, then most of the neutrons produced by the fission reaction get absorbed in ![]() before they escape out of the sphere and produce a chain reaction. Such mass of uranium in which one neutron, out of all the neutrons produced in one fission reaction, produces further fission is called critical mass. The volume of this mass of uranium is called critical volume.
before they escape out of the sphere and produce a chain reaction. Such mass of uranium in which one neutron, out of all the neutrons produced in one fission reaction, produces further fission is called critical mass. The volume of this mass of uranium is called critical volume.
If the mass of uranium is much greater than the critical mass, then the chain reaction proceeds at a rapid speed and a huge explosion is produced. Atom bomb works at this principle. If the mass of uranium is less than the critical mass, the chain reaction does not proceed. If the mass of uranium is equal to the critical mass, the chain reaction proceeds at its initial speed, and in this way, we get a source of energy. Energy, in an atomic reactor, is obtained according to this principle. The chain reaction is not allowed to run wild, as in an atomic bomb but is controlled by a series of rods, usually made of cadmium, that is inserted into the reactor. Cadmium is an element that is capable of absorbing a large number of neutrons without becoming unstable or radioactive. Hence, when the cadmium control rods are inserted into the reactor, they absorb neutrons to cut down on the number of neutrons that are available for the fission process. In this way, the fission reaction is controlled.
Nuclear fission and fusion (video)
What is Nuclear Fusion?
Such a nuclear reaction in which two light nuclei merge to form a heavy nucleus is called fusion reaction. We have known that the energy given out per nucleon per fission of heavy elements like that of uranium is 0.9 MeV. It is due to the fact that the binding energy per nucleon of the fission fragments is greater than uranium. In fact, energy is obtained from any nuclear reaction in which the binding energy per nucleon of the products increases.
Is there any other reaction besides the fission reaction from which energy could be obtained? In order to answer this question must ponder over again. This graph shows that the binding energy per nucleon increases up to A = 50. Hence when two light nuclei merge together to form a heavy nucleus whose mass number A is less than 50, then energy is given out. In the section on “Mass Defect and Binding Energy,” we have observed that when two protons and two neutrons merge to form a helium nucleus, then about 28 MeV energy is given out.
During a fusion reaction, some mass is lost and its equivalent energy is given out. In a fusion reaction, more energy per nucleon can be obtained as compared to the fission reaction. But unfortunately, it is comparatively more difficult to produce fusion. Two positively charge light nuclei must be brought very close to one another. To do so work has to be done against the electrostatic force of repulsion between the positively charged nuclei.
Thus a very large amount of energy is required to produce fusion reaction. It is true that a greater amount of energy can be obtained during a fusion reaction compared to that produced during a fission reaction but in order to start this reaction a very large amount of energy is spent. On the contrary, no difficulty is faced to start the fission reaction because neutron has no charge on it and it has to face no repulsive force while reaching the nucleus.
Let us take the example of fusion reaction when two deuterons are merged to form a helium nucleus, 24 MeV energy is released during this process i.e.,
![]()
But there is very little chance of the formation of ![]() nucleus by the merger of two deuterons. The probability occurring such a reaction is great one proton where one proton or one neutron is produced as given below:
nucleus by the merger of two deuterons. The probability occurring such a reaction is great one proton where one proton or one neutron is produced as given below:
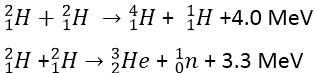
In both of these reactions about 1.0 MeV energy per nucleon is produced which is equal to the energy produced during fission. If ![]() and
and ![]() are forced to fuse then 17.6 MeV energy obtained I.e.,
are forced to fuse then 17.6 MeV energy obtained I.e.,
![]()
We have known that for the fusion of two light nuclei the work has to be done to overcome the repulsive force which exists between them. For this, the two nuclei are hurled towards one another at a very speed. One method to do so is to give these nuclei a very large velocity with the help of an accelerator. This method has been used in the research study of nuclear fusion of ![]() and
and ![]() . But this method of nuclear fusion for getting energy cannot be used on a large scale.
. But this method of nuclear fusion for getting energy cannot be used on a large scale.
There is another method to produce a fusion reaction. It is based upon the principle that the speed of atoms of a substance increases with the increase in the temperature of that substance. To start a fusion reaction the temperature at which the required speed of the light nuclei can be obtained is about 10 million degrees Celsius. As such an extraordinarily high temperature, the reaction that takes place is called thermo-nuclear reaction. Ordinary such a high temperature cannot be achieved. However, during the explosion of am atom bomb this temperature can be had for a very short time.
Until now the fusion reaction is taking place only in a hydrogen bomb. That extraordinary high temperature is obtained during the explosion of an atom bomb, due to this high temperature the fusion reaction between ![]() and
and ![]() sets in. In this way, a very large amount of energy is given out with the explosion.
sets in. In this way, a very large amount of energy is given out with the explosion.
A very large amount of energy can be had from a fusion reaction, but till now this reaction has not been brought under control like a fission reaction and so is not being used to produce electricity. Efforts are in full swing in this field and it is hoped that in the near future, some methods would be found to control this reaction as well.
Watch also:
READ ALSO:
Nuclear fission
Half life of radio active elements
Nuclear fusion
Radioactivity
Half life