Structure of atom for class 9 and 11
what is Atom?
“Atom is the smallest particle of an element that possesses the unique characteristics of that element.” All matter is composed of atoms; all atoms consist of electrons, protons, and neutrons except normal hydrogen, which does not have a neutron. Each element in the periodic table has a unique atomic structure, and all atoms within a given element have the same number of protons.
At first, it was thought to be a tiny indivisible sphere. Later it was shown that the atom was not a single particle but was made up of a small dense nucleus around which electrons orbit at great distances from the nucleus, similar to the way planets orbit the sun. Niels Bohr proposed that the electrons in an atom circle the nucleus in different orbits, similar to the way planets orbit the sun in our solar system. The Bohr model is often referred to as the planetary model.
Another view of the atom called the quantum model is considered a more accurate representation, but it is difficult to visualize. For most practical purposes in electrons, the Bohr model suffices and is commonly used because it is easy to visualize.
Our body is also composed of several trillions of atoms. A Greek philosopher Democritus gave the idea of the atom for the first time. Then, in the 19th-century john Dalton from England presented the first atomic model. According to him, all matter is composed of atoms. Atoms can neither be created nor destroyed.
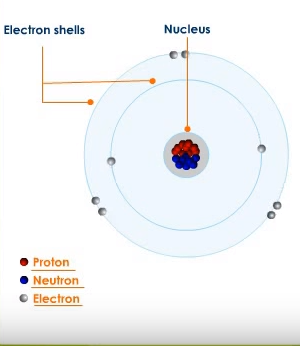
What are the three parts of an atom?
Is there any particle smaller than an atom? Sure, there is. Atoms are made of even smaller particles called electrons, protons, and neutrons. The central part of the atom is called the nucleus. Protons and neutrons are present in the nucleus.
Electrons
Electrons revolve around the nucleus. And electrons have a negative charge. Its mass is extremely small.
Protons
A proton has a positive charge. The number of protons in an atom is equal to the number of electrons revolving around the nucleus. It has a mass 1837 times greater than that of the electron.
Neutron
A neutron has no charge. This neutral particle is also found in the nucleus. The mass of a neutron is almost equal to the mass of a proton.
What is the charge on nucleus?
Nucleus has a positive charge because it consists of protons and neutrons, the charge on a proton is positive and on neutron is neutral means neutron has no charge. Therefore nucleus has a positive charge due to protons.
Why atom is a neutral?
Although electrons and protons in an atom have charges, it has no charge. In an atom, the number of protons is equal to the number of electrons. As a result, the total positive charge of protons balances the total negative charge of electrons. Because of it, the atom is neutral.
The Bohr Model:
An atom is the smallest particle of an element that retains the characteristics of that element. Each of the known 118 elements has atoms that are different from the atoms of all other elements. This gives each element a unique atomic structure.
According to the classical Bohr model, atoms have a planetary type of structure that contains a central nucleus surrounded by orbiting electrons. The nucleus consists of positively charged particles called protons and uncharged particles called neutrons. The basic particles of negative charge are called electrons.
Neils Henrik David Bohr (October 7,1885-November 18,1962) was a Danish physicist, who made important contributions to understanding the structure of the atom and Quantum mechanics by postulating the planetary model of the atom. He received the Nobel prize in physics in 1922.
Bohr drew upon the work or collaborated with scientists, such as Dalton, Thomson, and Rutherford, among others, and has been described as one of the most influential physicists of the 20th century.
Difference between atomic number and atomic mass with examples
Atomic Number
All elements are arranged in the periodic table of the elements in order according to their atomic number. The atomic number equals the number of protons in the nucleus, which is the same as the number of electrons in an electrically balanced neutral atom. For example, hydrogen has an atomic number of 1 and Helium has an atomic number of 2. In their normal or (neutral )state, the all-atom of a given element has the same number of electrons as protons; the positive charges cancel the negative charges, and the atom has a net charge of zero. The atomic number is represented by (Z).
Mass Number (atomic mass)
The sum of protons and neutrons in the nucleus is called its mass number. It is represented by A. The hydrogen atom has only one proton in its nucleus, its mass number is also 1. Carbon has 6 protons and 6 neutrons. Its mass number is 12. We can use atomic numbers and mass numbers to find the number of neutrons in atoms.
Mass number (A)=Number of protons(z) + Number of neutrons
Electrons in shells
We know electrons revolve around the nucleus of an atom. The paths of movement of electrons around the nucleus are called shells. Electrons are distributed in different shells. Shells are also called energy levels. These shells are labeled as K, L, M, N, O, P, Q, etc.K is the first shell. We can calculate the number of electrons in a shell using the formula:
Ne =2n²
Where ‘n’ is the number of shells.
The maximum number of electrons can be calculated in the 1st (k) shell as:
Ne= 2(1)²=2
The maximum number of electrons can be calculated in the 2nd (L) shell as:
Ne = 2(2)²=8
The maximum number of electrons can be calculated in the 3rd (M) shell as:
Ne =2(3)²=18
The maximum number of electrons can be calculated in the 4th (N) shell as:
Ne=2(4)²=32
The maximum number of electrons can be calculated in the 5th (O) shell as:
Ne=2(5)²=50
And so on for the next shells…
Why do atoms combine with other atoms?
Atoms combine with other atoms but they stop reacting with other atoms ( become stable ) when their outermost shell is complete having 8 electrons, or they have only one shell (k shell) with 2 electrons. For this purpose, an atom can lose, gain or share its electrons with other atoms. Two hydrogen atoms combine to form a hydrogen molecule (H2) by sharing electrons.
Related links
