Difference between conductors and insulators with examples
 The difference between conductors and insulators is that conductors are those materials that conduct electric current easily while insulators are those materials that do not conduct current easily. Copper, Silver, Gold, and Rubber are some examples of conductors and insulators.
The difference between conductors and insulators is that conductors are those materials that conduct electric current easily while insulators are those materials that do not conduct current easily. Copper, Silver, Gold, and Rubber are some examples of conductors and insulators.
What are conductors?
A material or an object that conducts heat, electricity, light, or sound is called a conductor. Metal wires are good conductors of electricity and offer less resistance to the flow of current. Why do metals conduct electricity?… Metals like silver and copper have an excess of free electrons which are not held strongly with any particular atom of metals. These free electrons move randomly in all directions inside metals. When we apply an external field these electrons can easily move in a specific direction.
This movement of free electrons in a particular direction under the influence of an external field causes the flow of current in metal wires.
Examples of conductors
- Copper
- Aluminum
- iron
These are some examples of conductors.
What are insulators?
A material that does not easily transmit energy, such as electric current or heat is called an insulator. why insulators do not conduct electricity? All materials contain electrons. The electrons in insulators, like rubber, however, are not free to move. They are tightly bound inside atoms. Hence, current cannot flow through an insulator because they are no free electrons for the flow of current. Insulators have a very large value of resistance.
Examples of insulators
- Glass,
- wood
- plastic
- fur
- silk
These are some examples of insulators
What is a Semiconductor?
A semiconductor material is one whose electrical properties lie in between those of insulators and good conductors. An example is Germanium and silicon.
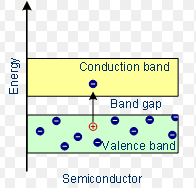
In terms of energy bands, semiconductors can be defined as those materials which at room temperature have:
- Partially filled conduction band.
- Partially filled valence band.
- A very narrow energy gap (of the order of ( 1 eV) between them.
At 0ºk , there are no electrons in the conduction band of semiconductors, and their valence band is completely filled. It means that at absolute zero temperature, a piece of Ge or Si acts as a perfect insulator. However, with an increase in temperature, the width of the forbidden energy band is decreased so that some of the electrons are liberated into the conduction band. In other words, the conductivity of semiconductors increases with temperature. It means that they have a negative temperature coefficient of resistance.
Related topics:
