examples of entropy in everyday life
Entropy measures how much thermal energy or heat per temperature. Campfire, Ice melting, salt or sugar dissolving, popcorn making, and boiling water are some entropy examples in your kitchen.
The concept of entropy was introduced into the study of thermodynamics by Rudolph Clausius in 1856 to give a quantitative basis for the second law. It provides another variable to describe the state of a system to go along with pressure, volume, temperature, and internal energy. If a system undergoes a reversible process during which it absorbs a quantity of heat ΔQ at absolute temperature T, Then the increase in the state variable called entropy S of the system is given by.
Entropy in thermodynamics formula
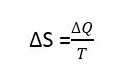
Like potential energy or internal energy, it is the change in entropy of the system which is important.
Change in entropy is positive when the heat is added and negative when the heat is removed from the system. Suppose, an amount of heat Q flow from a reservoir at temperature T 1 a conducting rod to a reservoir at temperature T 2 when T 1>T 2 . The change in entropy of the reservoir, at temperature T 1 which loses heat, decrease by Q/T 1 and of the reservoir at temperature T 2 , which gains heat, increases by Q/T 2. As T 1 > T 2 so Q/T will be greater than Q/T 1 i.e. Q/T 2 > Q/T 1.Hence:

It follows that in all-natural processes where hear flows from one system to another, there is always a net increase in entropy. This is another statement of 2nd law of thermodynamics. According to this law:
“If a system undergoes a natural process, it will go in the direction that causes the entropy of the system plus the environment to increase.”
It is observed that a natural process tends to proceed towards a state of greater disorder. Thus, there is a relation between entropy and molecular disorder. For example, an irreversible heat flow from a hot to a cold substance of a system increases disorder because the molecules are initially sorted out in hotter and cooler regions. This order is lost when the system comes to thermal equilibrium. The addition of heat to a system increases its disorder because of the increase in average molecular speed and therefore, the randomness of molecular motion.
Similarly, free expansion of gas increases its disorder because the molecules have greater randomness of position after expansion than before. Thus in both examples, entropy is said to be increased.
We can conclude that only those processes are probable for which entropy of the system increases or remains constant. The process for which entropy remains constant is a reversible process; whereas for all irreversible processes, the entropy of all systems increases.
Entropy examples
Every time entropy increases, the opportunity to convert some heat into work is lost. For example, there is an increase in entropy when hot and cold water is mixed. Then warm water in which results cannot be separated into a hot layer and a cold layer. There has been no loss of energy but some of the energy is no longer available for conversion into work. Therefore an increase in entropy means the degradation of energy from a higher level where more work can be extracted to a lower level at which less or no useful work can be done. The energy in a sense is degraded, going from more orderly form to less orderly form. eventually ending up as thermal energy.
For Related topics visit our page : Thermodynamics