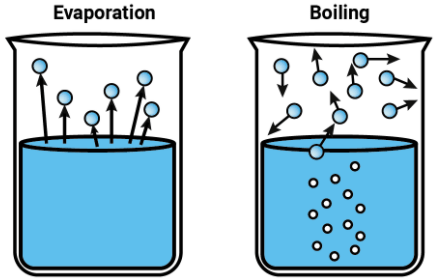
4 types of intermolecular forces in everyday life
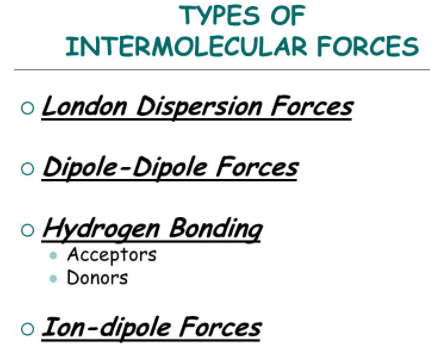
Forces between molecules are of electromagnetic origin. All molecules contain electric charges in motion. These molecules are electrically neutral in the sense that the negative charge of the electron is equal and opposite charge to the positive charge of the nuclei. This does not mean, however, that the molecules do not interact electrically.
For example, when two molecules approach each other the charges are distributed from their usual position in such a way that the average distance between opposite charges in the two molecules is a little smaller than that between like charges.
Hence attractive intermolecular forces results. These forces appear only when molecules are fairly close to each other. Thus these forces are short-range forces. The molecules repel each other because there is no way for a molecule to rearrange itself internally to prevent the repulsion of the adjacent external electrons.
Types of intermolecular forces
The intermolecular forces that act between the molecules are classified as:
- Permanent dipoles
- Induced dipoles
- Scattered dipoles.
- Hydrogen bonds
Within the 4 groups described above, the most relevant forces are the first 3 also known as Van der Waals forces.
Permanent dipoles
This type of union occurs when both molecules have positive and negative charges, that is, they are polar molecules or that have polarity, attracting each other electrostatically and forming the union.
Induced dipoles
This type of union occurs when a non-polar molecule redistributes the concentration of electrons (has the possibility of polarizing) when a polar molecule approaches, in such a way that a union is created between both molecules.
In this case, the polar molecule induces the creation of the apolar molecule in a polar molecule.
Scattered dipoles
In the latter case, the union occurs between nonpolar molecules that can be polarized, and when the latter occurs they attract each other creating the molecular union.
The junction that is created in this type of dipole has a very weak intensity and a very short life
The bonding energies generated by intermolecular forces are much lower than the energies generated by chemical bonds, but globally they are higher in number than the latter, playing a vital role in both the adhesion and cohesion properties of the adhesive.
Van der Waals —— 0.1 to 10 Kj / mol
Covalent Bond —— 250 – 400 Kj / mol.
The following table shows a comparison between the properties of intermolecular forces and chemical bonds:
| Intermolecular forces:
| Chemical links:
|
Graphical description of intermolecular forces
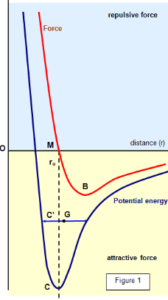
We can describe intermolecular forces graphically by considering the molecules spherically symmetrical. The figure shows how the potential energy of two molecules and the force between them changes with their separation. Here we can imagine one molecule to be fixed at O. The force at any point is found from F =-dU/dr, where U is the potential energy. Two forces act between the molecules:
- The repulsive force which predominates at short distances
- The attractive force which predominates at long distances
We can see from the graph that when the molecules are close to each other the repulsive force predominates, while at greater distances the attractive force is larger. The resultant force is:
- Repulsive from O to M
- Attractive from M to B but increasing with distance
- Attractive from B to infinity but decreasing with distance.
There is a position where the two forces balance, shown by M on the graph. This is the equilibrium position for molecules in the solid.
The potential energy is a minimum at this point. The separation distance between the two molecules at which the mutual potential energy is zero is called the distance of the closest approach. Any disturbance from this position would produce a force tending the return of the molecule to M.The force of attraction between the molecules increases as the molecules are separated from M to B. The breaking point is at B since beyond this point the force of attraction decreases with increasing separation.
For a molecule to be completely separated from its neighbor it must gain an amount of energy F, represented by CM on the diagram. The latent heat of vaporization for the two molecules is CM when there is no residual attractive force. This length also represents the latent heat of vaporization for the whole material. In a solid, the distance OM is some 2-3 ×10-10m and you can see that around this point the force between the molecules varies approximately linearly with distance.
Watch also a video: ( Intermolecular forces)
For Related Topics visit our Page: Thermodynamics