Difference between matter and energy
Matter and energy are two basic terms in Physics. The basic difference between matter and energy is that matter is the substance that has mass and occupies space, while Energy is the capability to do work.
If you want to learn more differences between energy and matter, then you are at the right place.
Keep reading …..
Matter Vs Energy: Comparative analysis
What the matter?
The matter is defined as everything that has a certain mass and volume and occupies a space of the existing space, and it includes all the things that come to the human mind. Water, air, clothing, bodies are some examples of matter. Solid, liquid, gas, and plasma are the states of matter.
The term “matter” derives from the Latin mater which means “mother”. This means that matter is the “mother” of everything around us. For example, air, although we cannot see it, is matter, because it is made up of molecules of nitrogen, oxygen, and other gases. The telephone, the computer, food, animals, buildings are all examples of matter.
See Also: States of matter
What are the characteristics of matter?
- It has mass: it is the amount of matter, for example, an electron has a mass of 9 x 10 -31 kg, a liter of water has a mass of 1 kg, the Sun has a mass of 1.9 x 10 30 kg.
- It has physical properties: within which density, electrical conductivity, melting or boiling point, volatility and hardness, among others, can be mentioned.
- It has chemical properties: matter can be transformed through chemical reactions, such as combustion, oxidation, decomposition.
What is Energy?
Energy is the ability to do work. It is the scalar quantity. SI unit of energy is Joule. Kinetic energy and potential energy are the basic types of energy.
What are the properties of energy?
- The amount of energy a body possesses is limited: the fruit on the table has an energy-limited by its mass and by the distance from the table to the floor.
- Energy is transformed into its different forms: the chemical energy of gasoline is transformed into kinetic energy when you move a piston in the car.
- There are various sources of energy, such as the sun, wind, and oil.
- It can be stored: chemical energy is stored in electrical batteries, in hydroelectric dams the gravitational potential energy of water is stored.
Read Also: Properties of matter
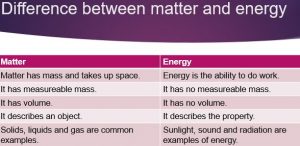
Difference between matter and energy in tabular form
| Matter | Energy | |
|---|---|---|
| Definition | That which serves as construction of nature. | Ability to do a job. |
| Constituents | Atoms, molecules, subatomic particles | Does not have |
| Types or forms |
|
|
| Unit of measure | Mass measurements: grams, kilograms, micrograms. Volume measurements: liters, cubic meters, milliliters. |
|
| Examples | Water, air, sand, stones, planets, computer, paper, plants, animals. | Light, heat, magnetism, microwave waves, electricity. |
Conclusion: Difference between energy and matter in points
- A fruit and a table are matter.
- the ability of the fruit to fall off the table and hit an animal is energy; the ability of the fruit to serve as food is energy.
- The capacity that a table (when burned) heats a room is energy; the ability of the table to break a window is energy.
