Thermal Expansion: Formula, examples and applications
Enter and Learn the best examples of thermal Expansion and Much more.
So, If you want to get Benefits from this post, you’ll love this post. This post also includes:
- Thermal Expansion Definition
- Thermal expansion Examples
- Applications
- Much more
Keep reading…
Thermal expansion definition
Most of the substances solids, liquid, and gases expand on heating and contract on cooling. Their thermal expansions and contractions are usually small and are not noticeable. However, these expansions and contractions are important in our daily life.
The kinetic energy of the molecules of an object depends on its temperature. The molecules of a solid vibrate with larger amplitude at a high temperature than at low temperatures. Thus, on heating, the amplitude of vibration of the atoms or molecules of an object increases. They push one another farther away as the amplitude of vibrations increases. Thermal expansion results in an increase in length, breadth, and thickness of a substance.
Let’s see the video now :
What is the difference between linear thermal expansion and volume thermal expansion?
Linear Thermal Expansion In Solids
It has been observed that solids expand on heating and their expansion is nearly uniform over a wide range of temperatures. Consider a metal rod of length L° at a certain temperature T°. Let its length on heating to a temperature T becomes L. Thus
Increases in length of the rod = ΔL = L – L0
Increase in temperature = ΔT = T – T°
It is found that change in length ΔL of the solid is directly proportional to its original length L°, and the change in temperature Δ T. That is
ΔL ∝ L0ΔT
ΔL =αL0ΔT …….(1)
L – L0=αL0ΔT
or L =L0(1+αΔT) …..(2)
Where α is called the coefficient of linear thermal expansion of the substance. From equation (1) we get
α = ΔL/L°ΔT
Coefficient of linear expansion
The coefficient of linear expansion α of a substance is the fraction increase in the length per kelvin rise in temperature.
See Also: Temperature Scales
Coefficient of linear expansion formula
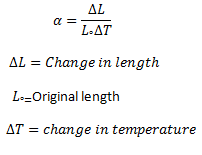
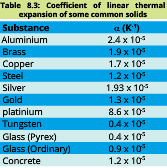
Below given table of linear thermal expansion of some materials:
Volume Thermal Expansion
The volume of a solid also changes with the change in temperature and is called volume thermal expansion or cubical thermal expansion. Consider a solid initial volume of V°. On heating, the solid to a temperature T, let its volume becomes V, then
Change in the volume of a solid ΔV = V – V°
change in temperature ΔT = T – T°
Like linear expansion, the change in volume ΔV is found to be proportional to its original volume V°, and the change in temperature ΔT. Thus
ΔV ∝ V° ΔT
ΔV=βV° ΔT ……(3)
V – V°=βV° ΔT
V = V°(1 + βΔT)
Where β is the temperature coefficient of volume expansion. From equation (3) we get
β = ΔV/V° Δ
Coefficient of volume expansion
The temperature coefficient of volume expansion β is the fractional change in its volume per kelvin change in temperature.
See Also: Radiant Energy
Coefficient of volume expansion formula
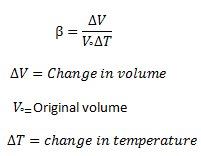
The coefficients of linear expansion and volume expansion are related by the equation:
β = 3 α
Values of β for different substances are given in table: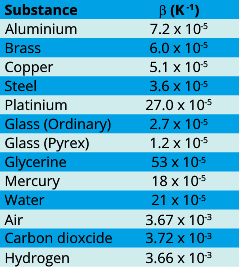
Consequences of Thermal Expansion
Why gaps are left in railway tracks? The expansion of solids may damage the bridges, railway tracks, and roads as they are constantly subjected to temperature changes.  So provision is made during contraction for expansion and contraction with temperature. For example, railway tracks buckled on a hot summer day due to expansion if gaps are not left between sections.
So provision is made during contraction for expansion and contraction with temperature. For example, railway tracks buckled on a hot summer day due to expansion if gaps are not left between sections.
Bridges made of steel girders also expand during the day and contract during the night. They will bend if their ends are fixed. To allow thermal girder rests on rollers in the gap left for expansion.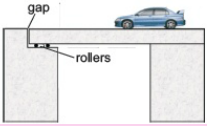
Overhead transmission lines are also given a certain amount of sag so that they can contract in winter without snapping.
Applications of Thermal Expansion in everyday life
Thermal expansion is used in our daily life.
Thermometers
In thermometers, thermal expansion is used in temperature measurements.
Removing tight lids
To open the cap of a bottle that is tight enough, immerse in it hot water for a minute or so. Metal cap expands and becomes loose. It would now be easy to turn it to open.
Riveting
To join steel plates tightly together, red hot rivets are forced through holes in the plates. The end of hot rivets is then hammered. On cooling, the rivets contract and bring the plates tightly gripped.
Fixing metal tires on wooden wheels
Iron rims are fixed on wooden wheels of carts. Iron rims are heated. The thermal expansion allows them to slip over the wooden wheel. Water is poured on it to cool. The rim contracts and becomes tight over the wheel.
Bimetallic Strip
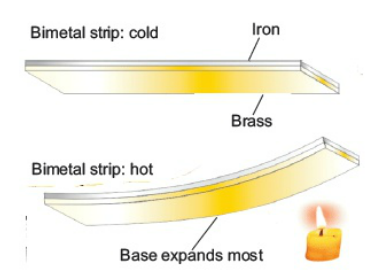
A bimetal strip consists of two thin strips of different metals such as brass and iron joined together. On heating the strip, brass expands more than iron. This unequal expansion causes the bending of the strip.
bimetal strips are used for various purposes. Bimetal thermometers are used to measure temperature, especially in furnaces and ovens. Bimetal strips are used in thermostats. A bimetal thermostat is used to control the temperature of the heater coil in an electric iron.
Thermostats
The thermostat is a heat-regulating device which works on the principle of thermal expansion.
Stay tuned with us to see applications of expansion:
Thermal expansion examples
Here Are Some Examples of thermal expansion in our Daily Life.
- Cracks in the road when the road expands on heating.
- Sags in electrical power lines.
- Windows of metal-framed need rubber spacers to avoid thermal expansion.
- Expansion joints (like joint of two railway tracks).
- The length of the metal bar getting longer on heating.
- Tire bursts in hot days when filled full of air due to thermal expansion.
Thermal Expansion of Liquids
The molecules of liquids are free to move in all directions within the liquid. On heating a liquid, the average amplitude of vibration of its molecules increases. The molecules push each other and need more space to occupy. The accounts for the expansion of the liquid when heated. The thermal expansion in liquids is greater than solids due to the weak forces between their molecules. Therefore, the coefficient of volume expansion of liquids is greater than the solids.
Liquids have no definite shape of their own. A liquid always attains the shape of its container in which it is poured. Therefore, when a liquid is heated, both liquid and the container undergo a change in their volume. Thus, there are two types of thermal volume expansion for liquid.
- Apparent volume expansion
- Real volume expansion
Anomalous Expansion of water
Water on cooling below 4 C° begins to expand until it reaches 0°C. On further cooling, its volume increases suddenly as it changes into ice at 0°C. When ice is cooled below 0°C, it contracts i.e, its volume decreases like solids. This unusual expansion of water is called the anomalous expansion of water.
Related Topics:
Show that temperature _40°c is unique in that it has the same numerical volume on the (F and °c) scale
It has a very good explanation
Love it, too helpful.