Nuclear Physics
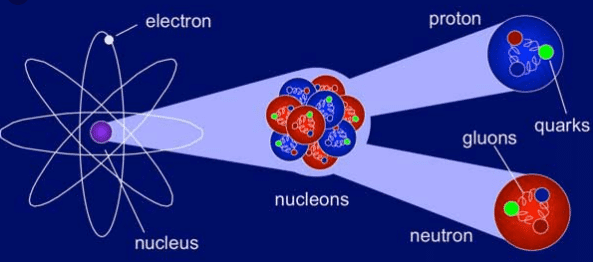 Nuclear physics is the study of the constituent particles of atomic nuclei, such as protons and neutrons, and the interactions between them. These interactions are able to hold the particles together at extremely small distances of the order of a few nanometers (10 -9 m). Some examples of phenomena studied by nuclear physics are radioactive decay, fission nuclear, nuclear fusion, etc.
Nuclear physics is the study of the constituent particles of atomic nuclei, such as protons and neutrons, and the interactions between them. These interactions are able to hold the particles together at extremely small distances of the order of a few nanometers (10 -9 m). Some examples of phenomena studied by nuclear physics are radioactive decay, fission nuclear, nuclear fusion, etc.
Introduction to Nuclear Physics
The nuclear physics studies the physical phenomena related to the core atomic, as power transitions, radioactive decay, fission, and nuclear fusion, among others. The study of nuclear physics involves the development of models that explain the functioning of atomic nuclei and their constitution, applications of nuclear energy in medical treatments, development of technologies for radiation detection ., new sources of energy, etc.
Nuclear Physics in Health
In recent years, Nuclear Physics has enabled, through nuclear medicine, the emergence of technologies of great impact on human health. A growing number of imaging studies have been performed using different types of radiation and particles.
In addition, a large number of patients receive cancer treatment through radiation produced by particle accelerators or natural sources of radiation, such as cesium-137, iodine-131, and others found in nature. There are now advanced cancer treatments with fewer side effects that are capable of destroying cancer-affected tissues through proton emission., neutrons, heavy ions, and ionizing electromagnetic radiation.
Some imaging studies are based on knowledge derived from research carried out by nuclear physics, such as computed tomography, nuclear magnetic resonance, positron emission tomography, single-photon emission computed tomography. These examinations provide richly detailed images of delicate organs and structures without the need for relatively high-security surgical interventions for patients.
Nuclear Physics and Environment
Nuclear physics is also widely applied to environmental studies: dating radioactive nuclei present in rocks and soil, for example, is of vital importance in determining the Earth’s past and defining climate patterns.
The earth’s atmosphere is constantly bombarded by highly energetic cosmic rays whose interactions with the carbon molecules in the air produce the carbon-14 isotope. This rare element has a half-life extremely long: every 5700 years, the number of this type of radioisotope present in living organisms, such as plants and animals, falls by half. In this way, it is possible to study the age of fossils and even determine the time when large forests or entire ecosystems stopped living.
See Also : Uses of radioisotopes
Nuclear Physics and Industry
Many techniques derived from nuclear physics, more explicitly from particle accelerators, have been used in industrial processes, promoting increased efficiency and great economic impact.
One of the most important applications for the industry is detectors used in determining the composition of semiconductor materials. Semiconductors are the raw material for all the electronics used, from chips in computers and cell phones to simple electrical connections. For these components to function perfectly, it is of fundamental importance that their purity is guaranteed. Thus, characterization techniques of chemical elements, such as PIXE (induced emission of X – rays particles), measuring the emission of X-rays of samples bombarded by protons during collision beam protons with atomic nuclei. These techniques also measure the emission of electromagnetic waves to determine characteristics, such as atomic mass and electrical charge, of some material.
See also: Alpha, Beta and Gamma Radiations
The PIXE technique and other techniques such as proton-induced gamma-ray emission ( PIGE ) are able to determine the exact composition of various sample types. They are also used in museums to determine the originality of work and in space probes such as Mars Rover, which aims to study the composition of the planet Mars.
Electricity production
Currently, about 11% of all electricity produced in the world comes from approximately 450 nuclear reactors. All nuclear energy is generated from the fission of heavy atomic nuclei, such as uranium, which becomes unstable after the capture of a slow neutron emitted towards them.
Since 2016, there are about 60 nuclear reactors under construction worldwide and another 150 planned. When implemented, these nuclear plants will account for 50% of the world’s power generation.
See also: How does a nuclear power plant work?
Sixteen countries rely on nuclear power to produce at least 25% of all their energy demand. France, for example, has 75% of all its energy produced by nuclear power plants due to the shortage of natural energy resources such as water potential, wind, geothermal, etc.
Nuclear power plants operate through chain reactions promoted by fissile elements, such as the uranium – 238 radioisotopes. In addition to the emission of particles, these elements emit electromagnetic waves that heat water to very high pressures and temperatures. When released, this water moves a large turbine (called a dynamo), generating an electrical current through the phenomenon of electromagnetic induction.
The Nuclear Physics course
To study thoroughly about nuclear physics, you usually need to graduate in physics and then specialize with a master’s or doctorate in the field. However, during the undergraduate course, nuclear phenomena are studied in the regular content (this may vary according to the menu of each course). In addition, it is possible to learn about nuclear physics through research carried out in undergraduate programs.
The most common Nuclear Physics-related contents in Physics courses deal with modern nuclear models, transitions of energy levels of atomic nuclei, radiation emission, radioactive decay, etc.
Formulas Used by Nuclear Physics
Check out some of the main formulas used in the study of nuclear physics and their uses:
| Formula | What is it for? |
| Mass defect | It calculates the difference between the mass present in several separate protons and neutrons and the mass of these combined particles: part of the total mass converts into binding energy. |
| Resting energy | Albert Einstein’s equation relating the difference in mass with the energy released during the nuclear fission of an element. |
| Radioactive decay | Lists the number of radioactive isotopes present in a given portion of matter. It can be used to calculate the age of a sample. |
| Alpha Emission | It lists, by means of energy conservation, atomic and mass numbers of radioactive elements before and after the emission of a helium nucleus during alpha emission. |
| Beta Emission | Lists the atomic and mass numbers of an element that emits a particle (beta) by conserving energy. |
| Gamma Emission | Represents the conservation of energy during the process of emission of gamma radiation. |
Nuclear Physics History
The history of nuclear physics began in the late nineteenth century and has been built until recent days. Check out a brief timeline with some of the most important milestones in Nuclear Physics:
1896 – Henri Becquerel, a French physicist, discovered that uranium salts are capable of staining photosensitive plaques, thus discovering the “uranic rays”.
1897 – Ernest Rutherford did research on “urânicos rays” of Becquerel and discovered the alpha and beta radiation, classifying them as to their electric charges and their penetrating power in the matter.
1898 – Marie Currie and her husband, Pierre Curie, discovered that “uranic rays” are also emitted by other elements (thorium) and proposed the term “radioactivity”. They discovered and named two new radioactive elements: polonium and radio.
1903 – Ernest Rutherford proposed to measure the geological age of the earth by detecting radioactive elements within it. In addition, he suggested that the earth is much older than previously believed. A few years later, the Earth was found to be about 4.2 billion years old. At the time, it was believed in a few hundred million years.
1906 Ernest Rutherford discovered that alpha radiation is actually the nucleus of a helium atom.
1909 – Undergraduate students Eugene Mardsen and Hans Geiger conducted the gold leaf experiment, in which a thin gold film is bombarded by alpha particles, which are reflected, indicating the high density of the atomic nucleus.
1930 – Paul Dirac met the fields of special relativity Albert Einstein to quantum theory and developed the equation of Dirac, anticipating thus the existence of antimatter.
1931 – Physicists debated possibilities for not conserving energy during beta decays. At this time, the Italian physicist Enrico Fermi proposed that, in this type of decay, the emission of two virtually undetectable neutral particles, which he called neutrinos, occurred.
1932 – Carl Anderson detected the existence of positrons, particles with opposite electric charge and mass equal to that of electrons.
1935 – Japanese physicist Hideki Yukawa proposed that the protons and neutrons in the nucleus of atoms are linked by a strong nuclear force, much more intense than electrical repulsion itself.
1938 – Otto Hahn and Lise Meitner discovered nuclear fission when bombarding uranium nuclei with neutrons.
1942 – Enrico Fermi was named chief scientist in charge of the Manhattan Project, intended to produce the first artificial nuclear chain reaction.
1945 – On July 16, the first nuclear bomb was detonated in New Mexico. Three months later, two atomic bombs were dropped in the cities of Hiroshima and Nagasaki, Japan, leaving more than 100,000 dead.
Related Topics
-
- Radiation and Radioactive decay
- Natural radioactivity
- Properties of alpha rays
- Properties of Beta rays
- Properties of gamma rays
- Radioactivity
- Artificial radioactivity
- Difference between natural and artificial radioactivity
- Half life of radioactive element
- Isotopes
- Radioisotopes
- Nuclear fission