Three types of radiations are emitted from radioactive elements that are Alpha, Beta, and Gamma radiations. This post also includes Properties of Alpha, Beta, and Gamma rays.
This post includes:
- Radioactivity Definition
- Natural radioactivity Definition
- Nature of Radiations
- Properties of Alpha Radiations
- Properties of Beta radiations
- Properties of Gamma Radiations
- Lots more
Keep reading…
What is radioactivity?
In March 1896 Henri Becquerel announced the discovery of radioactivity similar to X-rays. Although rays (X-rays) had been discovered less than four months earlier it was already known that X-rays come from the fluorescence wall of the discharge tube and thus it was thought that fluorescence and phosphorescence might be responsible for them.
Becquerel knew that Uranium salts became luminescent when exposed to bright sunlight, and he had heard that the phosphorescent radiation from the activated salts could penetrate opaque bodies. After studying these effects he found that a new type of radiation came from uranium salts whether these salts had been excited by light or not. He found that uranium salts that had been protected from all known exciting radiations for four months still emitted penetrating radiations without any noticeable weakening.
He recognized the parallelism between his discovery and the discovery of X-rays, and he found that the new radiations could discharge electrified bodies as X-rays do. He realized that the source of this newly discovered radiation was not fluorescence but the uranium metal. This property of Uranium, that it spontaneously emits radiation is called radioactivity.
After the announcement of Becquerel’s work piere and Madame Curie surveyed many other elements for such radiation emission and discovered that thorium showed a similar degree of activity. It was soon found that the radiation emitted consisted of three distinct types.
These were named α,β, and γ-rays for simplicity, and were found to be charged helium, fast electrons, and electromagnetic radiation of very short wavelength similar to x-rays respectively. Some artificial radioactive substances have also been made and the nature of the natural radioactive elements and artificial radioactive elements is the same.
What is natural radioactivity?
In 1896 Becquerel discovered that uranium and its compounds emitted some kind of radiation that affected photographic plates. This kind of radiation was named Becquerel rays.
The radiation emitted is the characteristic of the element and they are not affected by the physical or chemical state of the specimen.
“This process of spontaneous self-disintegration of some of the heavy elements is called natural radioactivity and the elements exhibiting this activity are known as radioactive elements. The well-known radioactive elements are uranium, thorium, radium, and polonium.
Nature of radiation
To study the nature of the radiation, a piece of a radioactive element, such as radium, is placed at the bottom of a small hole drilled in an LED block.
The emitted radiations pass through the hole and enter a vacuum chamber in which a magnetic field is applied perpendicular to the plane of the paper and directed away from the reader. The radiations are deflected by the magnetic field and form three separate images on the photographic plate placed above the hole in the chamber.
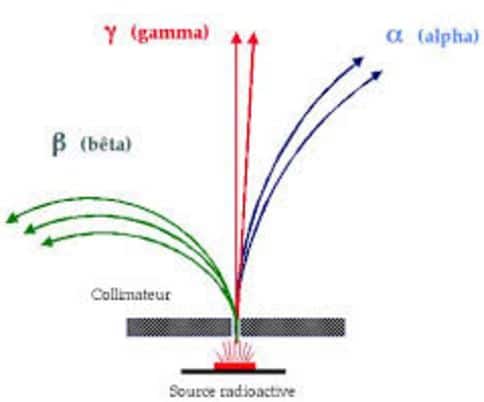
The radiations deflected towards the left are positively charged and given the name of α rays. The radiations deflected towards the right are negatively charged and given the name of β rays. The rays which are not deflected by the magnetic field have no charge and are called γ rays. Hence three types of radiations as mentioned above, are emitted by radioactive elements but an atom can never emit α and β rays simultaneously.
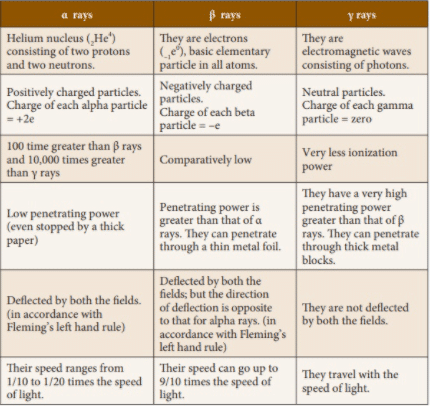
Properties of alpha α-rays
- They affect the photographic plate.
- The alpha rays are shot out from the radioactive material with large velocities. The velocities of alpha particles depend upon the radioactive substance is always the same.
- They have a low penetrating power.
- These rays are deflected by electric and magnetic fields showing that they are charged particles. The direction of their deflection shows that they consist of positively charged particles, each particle has charge +2e.
- When exposed to alpha rays the body suffers incurable burns.
- They produce an alpha heating effect.
- alpha rays have very high ionizing power.
- By measuring e/m it has been found that alpha rays consist of helium nuclei.
- When an alpha particle is emitted from a radioactive substance the charge number (z) and mass number (A) both change.
- They produce fluorescence in substances like zinc Sulphide.
Properties of beta rays
- They affect a photographic plate and their effect is greater than those of α-particles.
- They produce fluorescence in barium Platino cyanide etc.
- They are affected by electric and magnetic fields. Their direction of deflection indicates that they are negatively charged particles.
- By measuring e/m it has been found that beta rays consist of fast moving electrons.
- They can ionize the gasses but their ionizing power is much less than that of alpha rays.
- They can penetrate through a large thickness of matter.
- Beta rays are shot out from radioactive substances with very high velocities ranging from 1 % to 99 % of the velocity of light.
- When a beta particle is emitted from a radioactive substance, the charge number changes but there is no change in the mass number with the emission of a beta particle one of the neutron in the nucleus is converted into a proton.
Properties of gamma rays
- They have a high power of penetration and can pass easily through 30 cm thickness of iron.
- They are diffracted by crystals just x-rays.
- They possess the same velocity as that of light.
- They ionize the gas through which they pass but the ionization produced is very small.
- They affect a photographic plate and their effect is greater than that of beta rays.
- They produce fluorescence in barium Platino cyanide.
- They are not affected by electric and magnetic fields.
- When a nucleus emits gamma rays its charge number nor its mass number changes.
Difference between alpha beta and gamma rays in tabular form
| S.NO | Alpha Rays | Beta Rays | Gamma rays |
| 1 | These are nuclei of helium. | These are fast-moving electrons. | These are electromagnetic radiations. |
| 2 | They carry a positive charge. | They carry a negative charge. | They carry no charge. |
| 3 | They deflected towards the anode in an electrical field. | They are deflected towards the cathode in an electrical field. | They are not deflected in an electrical field. |
| 4 | Their velocity is approximately equal to 1/10th of the velocity of light | Their velocity is approximately is equal to the velocity of light. | Their velocity is equal to the velocity of light. |
| 5 | Their ionization power of gasses is very high. | These rays ionize gasses to lesser extent. | These are weak ionizer of gasses. |
| 6 | Their penetrating power is very low. | Their penetrating power is much greater than alpha rays. | Due to non-material nature and very high speed the penetrating power of these rays is greater. |
| 7 | Their mass is6.64 X 10-27 kg | Their mass is9.106 X 10-31 kg | They have no mass |
Related topics in our website are: